
…a nanoscientist’s quest to mimic Nature’s molecular blueprints
Have you ever found yourself entranced by the exquisite beauty and complexity of living things? Like the intricacies of a budding flower, or the mesmerizing patterns on a butterfly’s wing? Have you ever wondered: “what are living things made of?” Are these materials just as beautiful if we were to zoom way way in and look at the actual molecular building blocks that make up life? Take a look at the interactive link The Scale of Things to see just how small the building blocks of life really are! Well the answer is “OMG – totally!” All living things share a ubiquitous set of molecular building materials we call proteins, and they are absolutely stunning! They are not only smashingly beautiful to look at, they are capable of performing a mind-numbing myriad of very intricate and complex functions that are essential to life. In a very special guest post, leading nanoscience Professor Ron Zuckermann of the renowned Lawrence Berkeley National Laboratory recounts his life’s mission as a chemist to try and build artificial microscale sheets made up of nature’s very own building blocks—proteins. Everything you wanted to know about what nanotechnology is, exactly, why engineering proteins is the science of the future, and what we plan to use these discoveries for, under the “continue reading” cut.
I am a Materials Scientist working in a nanoscience research institute called The Molecular Foundry. A fundamental problem in nanoscience is how to make man-made materials with a similar level of precision and complexity at the molecular level found in nature. I am interested in applying lessons from the world of protein structure to practical man-made materials. If we are successful, we should be able to make materials that are cheap and rugged like a piece of plastic, yet be able to perform highly sophisticated functions, like recognizing a molecular partner with high specificity, or even catalyzing chemical transformations. Such materials could be used to make sensors for the detection of harmful chemicals, or as robust medical diagnostics that could survive harsh conditions, say in an underdeveloped nation. In a nutshell, we aim to make artificial proteins. This is an incredibly difficult problem, and one I have been working on for more than 20 years now. Sound a bit ambitious, or maybe a bit crazy? As I will describe in this article, it may actually be quite possible if we break the problem down into bite size chunks. The challenge comes down to two fundamental things: design and synthesis.

Protein architecture
When I look at the molecular structure of a protein molecule, I see an architectural blueprint that has survived untold generations of evolution and optimization. How then do we break open the hidden rules in these structures and use them to guide us in the design of man-made materials? Over the past several decades, scientists have used the biophysics techniques of X-ray crystallography and nuclear magnetic resonance spectroscopy (NMR) to determine the exact three-dimensional (3D) structure of thousands upon thousands of proteins. What’s cool is that these are all available for anyone to look at (for free!) and study in the Protein Data Bank.
The most fundamental thing to notice is that nearly all protein structures share the following characteristics: (1) they are made of a linear polymer chain that is folded into a precise 3D structure and (2) they are comprised of only 20 simple molecular building blocks called amino acids, arranged in an exact sequence along the polymer chain. When we think of a ‘polymer’ we think of a long chain of repeating chemical building blocks (called monomers) found in materials like nylon or polyethylene. Such man-made materials are incredibly useful and ubiquitous now in our environment (plastic bags or saran wrap, for example). But nature beat us to the punch a long, long time ago. Biopolymers, like proteins and nucleic acids are fundamentally way more sophisticated than man-made polymers. Even though they share the same basic architecture – a linear chain of chemical building blocks – biopolymers contain information encoded in their monomer sequence. This is not unlike the way we store information in a computer. But instead of a long string of 1’s and 0’s, nature uses long polymer chains of either 4 nucleotides (the building block units of RNA and DNA), or 20 amino acids (the building block units of proteins). These 20 chemically distinct amino acid building blocks are arranged in a particular order along the chain that we refer to as the protein’s “sequence.” This sequence is powerful because in many cases it provides all the information or “molecular instructions” necessary for the polymer chain to fold up into a precisely defined 3-dimensional structure. Once folded, the protein is poised and ready for action. The fields of Structural Biology and Protein Folding have revealed the exact way that proteins fold to form local “secondary” structures, called alpha helices and beta sheets, and how these assemble together to create the fully folded protein structure. Think all this sounds a bit too complicate? Try visiting FoldIt, a really fun video game where you can actually learn all about protein folding!
Protein Mimicry
If we ever hope to create man-made protein-like materials, it is safe to assume that we will need a polymer system that shares some of the basic protein-like characteristics: for example, they will need to have a sizable set of chemically diverse monomer building blocks that can be arranged in a particular order along a linear polymer chain of at least 50 monomers long. This is a quite a tall order simply from a chemical synthesis perspective. Moreover, once we are over that hurdle, design tools will be needed to help us figure out which sequences to make.
In the early 1990s, I invented a way to synthesize a new family of non-natural polymers we called “peptoids.” I had just graduated from UC Berkeley with a PhD in organic chemistry and joined a start-up biotechnology company to develop new technologies to accelerate drug discovery. We developed peptoids to be potential therapeutic drugs. The cool thing about peptoids is that the building blocks are very very close in structure to Nature’s amino acids, but different enough to be much more rugged. They can be made from very cheap and simple chemical building blocks, and they can be made in any predetermined sequence you want. We soon developed robotic synthesizers to automatically synthesize these materials for us (see below).

Before long we discovered that short peptoid chains (just 3 monomers long) could have potent biological activities and showed promise as drug candidates. But what really floored me was that the peptoid synthesis chemistry we developed worked so efficiently that we could link over 50 monomers together, one after the other. This means each building block was being attached to a growing chain with an incredible accuracy of over 99.5%! This was exactly the tool we needed to begin the quest for creating artificial proteins. We had discovered the most efficient and practical way to make non-natural polymers of a specific sequence. This was completely awesome!
There was only one problem – the company I worked for had no interest in such a bold quest into basic science. How could such a pursuit lead to a moneymaking product in a few months? In 2006 I moved to Lawrence Berkeley National Laboratory where I set out in earnest to search for artificial proteins. Fortunately, tackling difficult problems in basic science is much more the norm here. And more good news – my previous employer was kind enough to donate my robots to me. Armed with this technology to synthesize peptoid polymers, we turned to the next daunting task: which sequence of monomers should we make? It turns out that there are an absolutely astronomical number of possible peptoid sequences that can be made. Consider that there are several hundred building blocks to choose from at each of the 50 positions of a polymer chain. This means there are over 100 to the 50th power potential sequences to choose from. This is more than the number of atoms in the universe! What was a chemist to do?
Like Oil and Water
To help us focus our sequence design, we once again turned to nature. A long-time collaborator and friend of mine, Professor Ken Dill of UC San Francisco has studied protein structure in detail for decades, and has distilled some fundamental rules that are universal to all protein structures. He notes that protein structures are like globes with a water-loving surface and a water-hating (or oil-like) interior. The bottom line is you can basically lump each amino acid in the protein’s sequence into one of two categories: oil-like or water-like. This simple classification can tell you whether an amino acid is located on the inside or the outside of the protein.
The amazing thing about this insight is that it’s like looking at a protein wearing X-ray glasses! Instead of seeing 20 different “colors” of amino acids, we see only two: black and white. We are back to a simple binary code—like 1’s and 0’s. This makes it much easier to see the secret patterns hidden within the sequence. In fact, many researches have convincingly demonstrated that these binary patterns are simple, plentiful and predictable.
So with our handy X-ray glasses on we returned our gaze to the peptoid structure problem. We realized that all our sequence recipe needed was a touch of Dill! This meant we could greatly simplify our search for the right peptoid sequence. We needed to only consider two, diametrically opposed building blocks: water loving and water hating.
Nanosheets
Inspired by these insights, we set out to find the right sequence patterns that would result in a precisely ordered structure in a non-natural peptoid polymer. We began to systematically unlock the sequence code by using our robots to synthesize all the possible repeating patterns of these two disparate building blocks. We reasoned that if we were to make something precisely ordered, it would “crystallize” into something that we could see. Fortunately we have really powerful electron microscopes in my building. So we started cranking through all the possible sequence patterns, dissolving up each new sequence in some water and taking it down to the basement to look at them really close up.
Now it is a little nuts to think that we could make something precisely structured from a peptoid polymer, since it is known that each strand is about as stiff as a piece of overcooked spaghetti. And as one might expect, most of our sequences looked really messy and gooey. But very soon we saw something quite striking. In one particular sample, we saw large, flat paper-like objects with sharp, straight edges. And they were floating around everywhere in the solution. An unexpected sight to be sure!
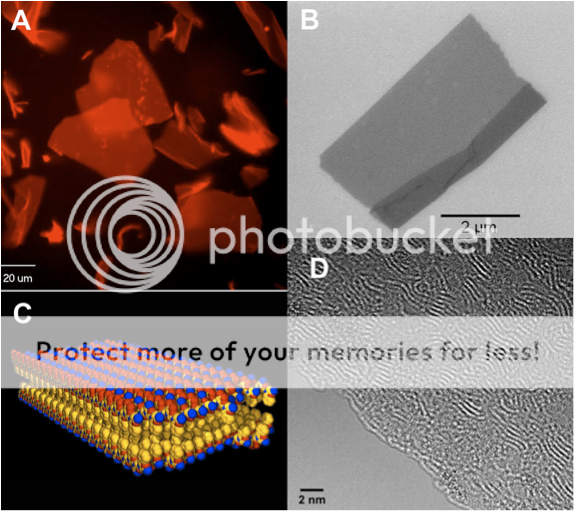
Fast-forward another year of making careful measurements and reproducing the results over and over. We determined that these sheets were only two molecules thick, and yet millions and millions of molecules wide. We had discovered the largest and thinnest organic crystals ever! We were able to use one of the most powerful electron microscopes in the world in the National Center of Electron Microscopy, to look directly at the individual polymer chains that make up the crystal. We could see these chains wiggle around and slide against one another as if they were alive. No one had ever seen such detail before – a truly awe-inspiring sight!
We were able to use many kinds of advanced analytical tools to tell us that these nanosheets were indeed very special. They have the same kind of ordered structure that a protein has: they have a defined inside and outside, and we know almost exactly where each atom is located in the structure. Essentially, we discovered the sequence code that programs the polymer chain to form a 2D sheet. No doubt there are more complex codes waiting to be discovered that will form even more sophisticated structures. This discovery was recently reported in the journal Nature Materials, and was picked up by the more mainstream publications WIRED.com and Chemical & Engineering News. These sheets are likely to be important for all kinds of potential applications. Their discovery is kind of like the invention of ‘molecular plywood’: a new kind of nanoscale building material from which we can engineer even more complex molecular architectures. It’s amazing what kind of beauty you can create from simple building blocks!
Basic research like this can move seemingly very slowly, which makes the occasional breakthrough like this all the more meaningful and exciting. It reaffirms for me that it is so important to listen to and cultivate your inner curiosity, surround yourself with like-minded people, and aim high. With enough patience and persistence, wonderful things await discovery!
Take a look at a brief video of Dr. Zuckermann explaining his lab’s nanosheet discovery:
Ron Zuckermann is the Facility Director of the Biological Nanostructures Facility at Lawrence Berkeley Laboratory. Dr. Zuckerman also provides numerous consulting services at the intersection of chemistry, biology and engineering.
*****************
ScriptPhD.com covers science and technology in entertainment, media and advertising. Hire our consulting company for creative content development.
Subscribe to free email notifications of new posts on our home page.
]]>
Last year around this time, ScriptPhD.com posted Breaking Bad, Chemistry Good, an in-depth article about AMC’s breakout hit Breaking Bad, and its stunningly accurate science content. Walter White, the show’s anti-hero, is a cancer-stricken high school chemistry teacher who starts cooking and dealing methamphetamine for financial security. In our article, we highlighted several clever uses of chemistry throughout the show’s run that not only integrated brilliantly into the plot but had realistic real-world applications as well. What a difference a year makes! Last week, Editor Jovana Grbić sat down with Breaking Bad‘s delightful Creator and Executive Producer Vince Gilligan to talk about the show’s origins, the science, and some behind-the-scenes secrets that will surprise even dedicated fans. We hope you enjoy reading our interview as much as we enjoyed chatting with him. The secrets of Breaking Bad, under the “continued reading” cut.
ScriptPhD.com: As a scientist (and PhD chemist in particular), Breaking Bad’s premise was very intriguing to me from the start. Can you share a bit about the inspiration for its origins?
Vince Gilligan: You know, Jovana, I wish I always had a better answer for where it all comes from. But I only can put a date to things—I only seem to remember when ideas came to me. In this case, Breaking Bad was an idea that came to me when I was speaking to an old college friend on the phone. He and I are both writers, and were both bemoaning the fact that—this was was about 2005—we were having trouble getting work and we were wondering where our next writing gig was going to come from. We thought instead, maybe we should switch occupations. Maybe we should buy an old RV and put a meth lab in the back and ride around in the Southwest. And we were joking around, but this character that became Walter White jumped into my head very quickly, right in the midst of this phone conversation. That’s when the idea came to me!

I have to say, as far as the science angle goes, I’ve always loved science, and yet I never had any real facility for it. I’ve always been a terrible student of mathematics in high school and in college. I’ve never even taken chemistry, I hate to admit it. But I’ve always loved science. And for most of my life, I’ve been a subscriber of Popular Science magazine and Popular Mechanics. But I’ve never made the jump from Popular Science to Scientific American! I don’t have a deeper understanding, but I love the idea that there are real concrete black-and-white answers in science and math, that we don’t unfortunately get in the rest of our life. The world, and our lives, is full of gray areas and uncertainties and opinion versus fact. And yet in mathematics, 2+2=4 and always has and always will. In science, certain chemicals put together in a certain way always create the same compound. I love that idea about science and I always have. I just wished in my heart of hearts that I had a deeper facility and understanding for it, but I have to say it is fun to live it by proxy and to write about a man who has a very profound understanding of chemistry and of science.
SPhD: I find it ironic hearing you say that, because a lot of people who are far less humble about their scientific capacity, their shows are far less accurate to incorporate it. Breaking Bad is one of the few shows or films that we have given a top-notch grade to for scientific accuracy and plausibility, allowing for some Hollywood liberties, of course. Who are some of the people that you consult with to write the chemistry experiments and tidbits, and what has been the general reaction within the science community?
VG: We have a lot of really good help. We have a woman named Dr. Donna Nelson, and she is the Associate Professor of Chemistry at the University of Oklahoma. When Breaking Bad first went on the air, she contacted our office pretty early on (a few episodes in) and she said she liked what we were doing and if she could ever be of any help to us, she’d be happy to be a resource. And we have taken her up on that offer, and she has been a resource to us over the seasons. We run certain moments that happen in the story by her is as accurate as possible, and she’s been a great help. I know Bryan Cranston [Walter White], our star, shadowed a UCLA chemistry professor for a week or two before he shot the pilot, to immerse himself in the world of chemistry, not just to learn a bit about chemistry, but to see what a prototypical chemistry professor looks and sounds and acts like. Also, a part of my original inspiration, to be fair, was my long-time girlfriend Holly, her brother Hank, who I borrowed the name for Walt’s brother-in-law from, got a PhD in chemistry while he was in the Navy and is a chemist for a government organization out East and is studying red tides off the Atlantic coast. I also cannot leave out a man named Dr. Victor Bravenec, and he’s a DEA chemist based out of Dallas. I have to give great credit to the DEA, because they have been helpful since Day 1 on our Pilot, and Dr. Bravenec in particular has been helpful with the chemistry of methamphetamine and the particulars of how it is created in a laboratory. I’m so proud of the fact that you’re reacting to how realistic the chemistry is on our show, and so much of that is due to our wonderful advisors.
SPhD: One of the things I love most about Breaking Bad is that chemistry acts like an integral character, one that plays a very important role in Walt’s identity, the cooking and production of the meth, and in some cases, their very survival in some pretty hairy situations (as in the case of the fulminated mercury and homemade battery). Was it always the plan to have it be such a big part of the show or is that something that evolved?

VG: I did always like the idea that this would be a cable TV version of MacGyver. That was not first and foremost when I was coming up with the idea. First and foremost to me, the show is a character study about a man who is undergoing a radical transformation. He’s transforming himself from a protagonist to an antagonist. The whole show, in that sense, is an experiment to continue chemistry analogy. But there was always an element that I thought we could have fun with is the MacGyver aspect, as it were and the idea of using [Walt’s] knowledge to get him out of a jam every now and then. And I have to confess, as much fun as we have with that stuff, the show has taken so many dark character turns as he progresses from a good guy into a bad guy that of it goes by the wayside, although not intentionally. But those moments you speak of were a lot of fun to come up with and a lot of fun to write, and I’m hoping we’ll find a way to insert more of those moments as the season progresses.
SPhD: You’ve brought it up, so let’s just talk about where Breaking Bad goes from here. To me, Season 1 was very much about the construction of these characters, their situation, the “building up” of their individual new worlds. In many ways, Season 2 was the de-construction, where each character felt something fall apart in some way; Walt’s lies and second life, Jesse’s addiction and girlfriend’s death, Skyler’s trust in Walt, and possibly Hank’s DEA career. What do we look forward to in Season 3?
VG: Good question. And I hate to admit it, but I’m not the best at looking forward. You know the old expression “You can’t see the forest from the trees?” We are so deep into these characters’ lives that sometimes, I myself and my writers are not the best people to ask for a complete bigger picture explanation of what in fact is going on. All I know for sure is that Walt is in the process of change and transformation, and it’s an interesting experiment, one that we’re doing for the first time, as opposed to a repeated experiment. We can’t even predict the outcome!
SPhD: What’s funny is seeing the first few episodes of Season 3, I get the feeling Walt misses the badness and the danger of it all. He wants so badly to be good and to repudiate this person he’s become, and yet, I get the feeling he misses some of the perks and the thrills that it brought. Am I completely off-base here?
VG: No you are not off-base at all. That’s very astute. He’s an interesting character, because a lot of what this show is about to me is the human capacity for rationalization. We all rationalize our behavior to ourselves, our ideas, our actions. It’s just something that human beings do, it’s part of us. We usually do it in small, minor, insignificant ways. But Walt takes that to the nth degree. He stretches that to the breaking point. He’ll go around saying to himself, and when pressed by others to them, that this criminal behavior he finds himself engaged in is done purely to aid his family. He does it for their financial benefit. And yet we put the light on that pretty early in Season 1. If you’ll remember, he gets this offer from his rich former lab partner for a great job that sounds like it’s no strings attached and with all the money that he’ll need to treat his cancer and for his family to be financially solvent. And lo and behold, he doesn’t take it. That’s one of the moments I’m most proud of on our show, because prior to that moment, the show could have gone in a very different direction—we could have had the show become a weekly procedural. This week the meth lab burns down, and next week his money gets eaten by rats, and the next week after that the police arrest him, but he has to weasel out of that.
But early on we all recognized that a show like this can’t reach its full potential if we don’t really examine this guy at a deeper level. A lot of people, millions of people, especially with the economy being in the toilet, face similar situations, unfortunately. People face the issues that Walt faces, where money is a real problem. And most people don’t decide to cook crystal meth! So we realized early on that Walt has to have some kind of darker component to him that he’s perhaps always had. This is a man who is, above all else, prideful. And he bursts with pride at this avocation that he’s taken up, and he’s prideful about his product, about its quality. In so many ways, he’s just a sad little man who has felt passed over his whole life. He feels like he hasn’t really existed until he’s become a criminal, until he’s broken bad.
SPhD: My favorite episode to date has been the Season 2’s Peek-a-Boo, and its exploration of the beautifully complex character of Jesse Pinkman. His filial relationship with Walt is a cornerstone of this show, and has rather ironically blossomed amid the self-destruction of Walt’s relationship with his family and Jesse’s estrangement from his own parents. Can you talk about their relationship and your take on how these two individuals fit in each other’s worlds?
VG: Absolutely! It is a very interesting relationship, and it’s one that I didn’t realize what it would become. I’d love to say I knew from Day 1 when I was writing the pilot what this show would be, exactly, and all its dynamics, but the truth is that they’ve been a work in progress. A lot of this grows and creates itself, and the actors and writers bring so much to it, and it’s amazing to stand back years later and realize what you’ve had a hand in creating. But yes, it’s very much a father-son relationship between Walt and Jesse. And I have to say, as further evidence that this wasn’t the original intention of the show, my intention was to kill Jesse off at the end of Season 1. He was going to be kind of a character who helped Walt get into the business, and then got violently killed in a drug deal gone wrong. And the whole point of his existence would have been to get Walt into the business and then give Walt a reason to feel very, very guilty, further fueling bad behavior on his part. Luckily, we hired Aaron Paul to play Jesse, and he is such a fine young actor and so talented and charismatic that very quickly on, like in Episode 1 or 2, I realized that there was no way I was killing him off.
SPhD: And where do they go from here? They’re in a tenuous place right now. Together but apart, not really sure where things are going.
VG: It’s interesting, you put your finger on quite a lot of it. And it’s very much a love-hate relationship between these two guys, but it’s mostly love-hate on the part of Jesse. There are times when I’m not sure Walt has any regard for Jesse at all. Very often, there’s a coldness to Walt, and a desire just to get the job done and he sees Jesse as a useful tool but not much else. He doesn’t seem to have much regard or respect for Jesse, so every now and then, when he throws Jesse a scrap of respect, you feel how thirsty Jesse is for that. It’s funny, Jesse is sort of the moral center of our show. Even though he started off in the business before Walt, and Walt was a straight-arrow character before we meet Jesse, somehow Jesse seems to have a more refined and defined moral center. Very often, Jesse is the one who says “Should we really be doing this? Aren’t we being greedy? Haven’t we made enough money already?” He really is the moral center of the meth trade that we portray on the show, and Walt, who should be a father figure to him, who should be ushering Jesse out of the business, and a large part of us as the audience wishes that he would do the right thing. We know in our hearts that he most likely never will.
SPhD: They’re all beautiful relationships and I can’t wait to see where they all go. I love that you’ve even made me hate Skylar [Walt’s long-suffering wife], which I never thought I would do. I’m so sorry for that!
VG: It’s so funny, and I’m glad you brought that up, because you are definitely not even close to being the first person to say that. It’s just a funny thing about storytelling. Even when your hero is not a nice person, and he’s doing bad things, because he’s your protagonist, if you’re along for the ride, then you start to see the world through his eyes. It’s the nature of storytelling. It’s the same thing when you’re watching The Sopranos or some other show where there’s someone who is pretty much a reprehensible character is nonetheless your protagonist. You’ll root for that person to succeed. And right now, Skylar gets in the way, on a purely mechanical level, of Walt’s success and happiness and therefore we see her as an obstacle and we don’t like her for what she’s doing. So you’re not alone in feeling that way. But the funny thing is, I see Skylar as the good guy and Walt as the bad guy. I love Walt! He’s a great guy to write for, but he’s kind of a monster when you think about it. He shouldn’t be breaking back into the house, trying to get back into her good graces when the things he’s done and the lies he’s told really make him not the good guy. She’s being heroic when she doesn’t tell the police on him.
SPhD: Thanks, Vince, and we really appreciate you joining us!
VG: Thanks, and it really makes me feel good to hear that you think the science is authentic, because we do our best, and we will continue to.
Interested in watching the show? Start with this brilliant six-minute catch-up video:
Season 3 episodes of Breaking Bad air Sundays at 10 PM on AMC.
~*ScriptPhD*~
*****************
ScriptPhD.com covers science and technology in entertainment, media and advertising. Hire our consulting company for creative content development.
Follow us on Twitter and our Facebook fan page. Subscribe to free email notifications of new posts on our home page.
]]>
All right class, settle down, settle down. My name is Mr. Ross, but you may call me BR. Welcome to Pop-Culture Science 101. I know what many of you are thinking: “Science is boring; I just don’t get it.” I can understand those sentiments. But that’s only because of the ways you’ve been taught in the past. Today is going to be different. On this, the third day of the Science Week collaboration between ScriptPhD and CC2K, we decided to have a bit of silly fun and cover a couple of traditionally esoteric science topics from an angle I doubt any of you have considered before—pop culture icons. So get out your notebooks and pens, today’s lesson begins now! Please click “continue reading” for more.
Lesson 1: Genetics/Evolutionary Theory
It’s easy to get intimidated if you hear terms like Neodarwinism and Mendelian genetics. If I say deoxyribonucleic acid polymerase, well, I might as well be speaking Swahili, right? Heck, you might not even know who Charles Darwin was, or why his work is so important to biology. Doesn’t matter. Forget the technical jargon; don’t worry about the minutiae. The essentials of genetics and evolutionary theory can be learned simply by considering the X-Men. Yes, I mean the comic book superhero team.

The X-Men are superheros not because they’re aliens or because they were exposed to some kind of radiation, but because they were born as such. They bear mutations in the so-called X gene (yes this is make-believe, but for the purpose of today’s lesson it will work just fine). A gene is nothing more than a unit of information in your DNA that instructs something. Eye color. Hair color. How tall you are. Basically everything that makes us unique (and that makes us human, as opposed to some other species) is because of our genes. In the case of the X-Men X gene, mutations herein can bestow superpowers, though, this isn’t always the case.
See, mutation is a random thing. Mutations in genes can be negative as well as positive, debilitating just as often as advantageous. The key here is to consider how a mutation affects an organism’s fitness to survive. Ah, did that ring some bells? Some of you comic book fans might be thinking of the X-Men’s arch-enemy Apocalypse and his fanatic devotion to the concept of the “survival of the fittest.” The man (or mutant, rather) is a strong proponent of Darwin and his theory on evolution by natural selection. I know that might sound complicated, but it’s really not. Let’s go back to the X-Men. Consider the following:.
Let’s compare Wolverine with some average mutant Joe Schmoe off the street. Wolverine’s X gene mutation grants him numerous super powers including enhanced strength, speed, senses, claws, and an extraordinary healing ability. Joe Schmoe (for the sake of argument) has a mutation in the X gene that has bestowed him with, let’s say a prehensile tail (i.e. a tail like that of a monkey). That’s it. He’s not super strong, he can’t climb trees particularly well, he’s just a guy with a tail.
Now for the selective pressure. Senator Kelly teams up with some other unsavory types in the government and creates the Sentinels, giant robots programmed to detect and eliminate mutants with extreme prejudice. I ask you, who will have the better chance to survive? Who is more fit? Joe Schmoe, or Wolverine? Obviously Wolverine. Chance has given him a mutation that makes him more likely to survive a selective pressure (the Sentinels) than our pal Joe, hence Wolverine is more likely to reproduce and pass on his mutation to his offspring. That, friends, is evolution.
Lesson 2: Acid-Base Chemistry

One of my favorite chemistry teachers growing up was a mullet-coiffed action escape artist by the name of MacGyver. His lessons were so memorable because they were practical applications of chemistry using common household items to resolve extraordinary situations. Granted, the majoritysome of his lessons exaggerated the bounds of what was truly possible (sometimes Mac was more about style than substance), but often his lessons were grounded firmly in reality.
Take, for example, the time he demonstrated how to make a fire extinguisher from simple items found in an ordinary kitchen, and gave a lesson on acid-base reactions in the process (episode 132, “Good Knight MacGyver”).
Let’s say for the sake of dramatic storytelling (this IS a science in entertainment site, after all!) that your parents are going out of town, and you’ve decided to have a few friends over (including Dylan, that totally cute guy in your English Lit. class you’re hoping will ask you to prom). You decide that in order to elevate your little party above run-of-the-mill get-togethers you’re going to make your very own mozzarella sticks and jalepeno poppers. You fill a pot with vegetable oil and turn the burner all the way to “hi”. You’re ready to do some serious frying.
But then you get distracted, and the oil heats to the point of ignition. Your attention is pulled away from your cell phone by smoke coming from the kitchen. You have a grease fire to deal with. If you burn your parents’ house down they’ll ground you until you’re 30. You have to act fast. You quickly turn off the stove burner, and you know that if you throw water on the fire it will just make it worse. You could put it out if you had a fire extinguisher, but your parents don’t keep one in the house. Then you remember MacGyver’s chemistry lesson.
Acting quickly, you open the fridge and pull out the half-full bottle of Diet Coke and the box of baking soda your mom put in there because the Arm & Hammer commercials assert it will help remove unwanted odors. Then you pull out the large bottle of white vinegar from under the sink that hardly ever gets used. You pour the Diet Coke out onto the floor and fill the 32-ounce Coke bottle with as much baking soda as you can get in there (you notice it’s almost 3/4 full). Now comes the tricky part.
You quickly pour vinegar into the bottle, cover the opening with your thumb, give it a quick shake, and point it at the fire. Bubbly liquid begins to shoot out of the mouth of the bottle. You sweep the bottle back and forth, noticing with relief that the bubbly liquid is putting out the fire. There’s some smoke damage and one heck of a mess to clean up (and that party is definitely canceled), but catastrophe has been averted!
As to why this works, let’s open up our Unofficial Macgyver How-To Handbooks to Chapter 3, page 70:
Fire extinguishers work by removing one of the critical ingredients for a fire: oxygen. When vinegar is combined with baking soda, the two react and produce carbon dioxide gas or CO2. This type of reaction is known in chemistry as an acid-base reaction, and the vinegar (the acid) and baking soda (the base) model is a high school chemistry classic:
NaHCO2 (baking soda/base) + CH3COOH (vinegar/acid) –> CO2 (gas) + H2O (water) + Na (Sodium) + CH3COO (a combination of hydrogen, carbon and oxygen that we like to call “magic dust”)
The CO2 gas produced by this reaction has a heavier molecular weight than does the surrounding air, which is comprised primarily of nitrogen and oxygen, so the CO2 sinks into the bottom of the room. As the reaction continues, more and more carbon dioxide gas is produced and slowly fills up the room, displacing the oxygen. When the level of carbon dioxide has risen to the level of the flame, the flame will go out from lack of available oxygen. As Mother always said: “If I’ve told you once, I’ve told you 1,000 times, any combustion-reaction (fire) requires a primary oxidant (oxygen) in order to work.” Yeah, she’s a special lady, our mom.
A video demonstration:
Class dismissed.
“A Pop-Culture Science Lesson” is an original article by CC2K games editor Big Ross.
Science Week 2010 is a collaboration between ScriptPhD.com and CC2K.
*****************
ScriptPhD.com covers science and technology in entertainment, media and advertising. Hire our consulting company for creative content development.
Follow us on Twitter and our Facebook fan page. Subscribe to free email notifications of new posts on our home page.
]]>
He is one of the most popular and explosive (sometimes literally!) science columnists of our day. Since 2005, he has written the Popular Science blog Gray Matter. He has been willing to try virtually any chemistry experiment known to man, all in the interest of proving a theory and educating (and entertaining) a fortunate lay audience. He has created the most widely acclaimed periodic table ever, which has been replicated into posters, an actual table, playing cards, and now, a gorgeous full-color hardcover book. Who is this mad scientist I am referring to? Why, Theodore Gray, of course! For Day 3 of Science Week, ScriptPhD.com is thrilled to review his new book The Elements, an equal parts homage to chemistry and photography. Editor Jovana Grbić sat down with Theo in a candid, in-depth interview about his books, his favorite elements, and the responsibility science writers have to informing the public. More more content, please click “continue reading.”
It is a staple of every high school and college science class. Its familiar shape has stayed largely the same since 1869, when Siberian chemistry professor Dmitry Ivanovich Mendeleev first grouped chemicals together according to similar properties. It is indispensable to scientists of all disciplines, and has even inspired our very own ScriptPhD.com logo! It is, of course, the periodic table of the elements. There is an immense challenge in taking such a well-known, immutable scientific entity and making people see it in a way it has never been seen before. And to do so using photography and creative writing? To the moon, Alice! Yet in Theo Gray’s gorgeous new book The Elements, this is precisely what occurs—a rebirth for ruthenium, rhodium, radium, rubidium… and the rest. Each page (as seen below) is elegantly laid out with gallery quality photography, sometimes of compounds, minerals and applications that would surprise you, and fascinating stories that turn each element into its own unique chapter in the hallowed Bible of chemical history.

Chemistry is the central science. But as you get to know the periodic table better while reading The Elements, it quickly becomes apparent that chemistry is also a science with much character. It is smelly, as is the case with sulfur. It’s sometimes a mouthful, as in the longest element name, praseodymium. It helps us control mood swings, as is the case with lithium. It helps us when we’ve overdone it at the weekend barbeque, as is the case with bismuth subsalicylate. Perhaps you know it better as Pepto-Bismol. Chemistry helps doctors and scientists to save lives. A radioactive isotope of technetium is naturally bone-seeking, and helps radiologists diagnose everything from compound fractures to cancer. Should you ever need to get an MRI, chances are you’ll have to consume a contrast dye composed of the element gadolinium. Sometimes, a fickle element both saves and takes lives. Chlorine, for example, has saved hundreds of millions of lives as an antiseptic and disinfectant in small quantities, yet is poisonous in large quantities. Likewise, selenium is an essential nutrient, but toxic in large doses. Gray also pays tribute to a sullied, marginalized element that has gotten a bad rap over the years: zirconium. If you’re considering proposing to that special someone, zirconium, like the overpriced aged carbon of which it is a replica, is near the top of the hardness scale and equally as beautiful for a fraction of the cost! On an unrelated note, thallium is one of the most effective poisons on the periodic table, owing to common symptoms that few doctors can pinpoint. For utter destruction on a wanton scale, however, one need look no further than uranium:
What is most unique and attractive about The Elements is the lengths to which it goes to envelop artists and creatives in the world of science, many of whom would be surprised how much of their craft they owe to chemistry. Iconic photographers like Ansel Adams would be nothing without magnesium, which has been widely used in camera flashes to provide light. For you font and graphic design nerds out there, one thing the typeface documentary Helvetica omitted is the magic that lies in the combination of antimony, lead and tin—it expands when solidified from a molten state. Voila! 650 years (and counting) of movable typeface. Film buffs who have enjoyed movies like Avatar (and the upcoming Hubble 3D) on IMAX would be interested to know that IMAX projectors use 15 kW short-arc Xenon projector lamps. Jimmy Hendrix, Stevie Ray Vaughan, Eric Clapton, ZZ Top, BB King, and countless other guitar legends would be silenced were it not for the obscure element samarium, which in combination with cobalt is used as a magnet for electric guitar pickups. And for all of you DJs and musical purists who agree with The ScriptPhD that no musical sound is sweeter and sharper than that of a vinyl record, did you know that phonograph needle tips are made of osmium?
Interview With Theo Gray

ScriptPhD.com Editor Jovana Grbic was fortunate to chat with Theo Gray recently, and was eager to get his perspective on the making of The Elements and Mad Science books, and some more meaty science policy issues as well.
ScriptPhD.com: Tell me a bit about the idea to put together The Elements.
Theo Gray: The book goes back quite a ways. I started collecting elements, really by accident, in 2002. We needed a table for the common area of the office I was moving into, and I didn’t want some kind of an ugly thing that you could get from an office supply catalog, so I had tables on my mind. Then, I was reading Uncle Tungsten by Oliver Sachs, and he describes a periodic table that he used to visit at the science museum. I took that literally, and thought the table literally looked like a table, and that seemed like the coolest thing—someone should make a periodic table [with slots for the elements], and at that point no one ever had! Because of the way the table was designed, it was easy to make little compartments underneath each element where you could put a sample of the element. This was right around when eBay was exploding, and it turned out you could get a great many elements there. It kind of happened by accident. One table led to another, and pretty soon, I had thousands of element [samples], pure and industrial compounds. Then I started photographing them to remember what they are. I put the cataloged list and accompanying photographs on the web, and people liked it, and the Ig Nobel Award I got in 2002 made me think that this is something other people might be interested in, too.
One thing led to another, and the cameras started getting fancier, and the photography started getting better, and pretty soon I thought I could make a poster [of the elements]. This poster is now seem on TV shows from MythBusters to Hannah Montana, which eventually led to The Elements.
SPhD: One of the things I really loved about The Elements was the humor interspersed throughout the copy. You manage to really make it fun, lively and at times hilarious. Was this writing style meant to parallel how you view science, and chemistry in particular?
TG: The other thing that I’d been doing [while compiling the book] every month, was writing a column for Popular Science Magazine, Gray matter. And it’s popular, it’s a popular magazine, as the name implies, written very much for a lay audience. So I did that every month, writing 350-450 words about a certain topic of science and working with excellent editors there to refine the language to compete with other popular publications—immediately engaging, and with some fun in it. I think it’s fair to say that over the course of the five years that I’ve been writing the column, I’d refined my ability to write consicely about a focused topic. And that’s what The Elements book is, a hundred Popular Science columns all wrapped up. In some cases, the story behind an element was so much more interesting than any practical uses we could highlight. [Editor’s note: radium is a particularly good example of this!]
SPhD: What were the biggest challenges during the making of the book and photography of the elements?
TG: In terms of the writing of the book, the rare earths, or Lanthanide series, was the toughest by far, because all of the Lanthanides are very similar [in chemical property]. In many cases, for commercial applications, they’re essentially interchangeable. If you’re making lighter flicks, it really doesn’t matter what rare earth you’re using—and they don’t even purify them, they just take them out of the ground. And many of them don’t really have any interesting applications. Although, it was interesting to discover Thulium (Tm 69), for example, which I’d gone for years thinking that no one actually cared about. But it turns out in the lighting industry they care passionately about thulium, and they couldn’t live without it [for metal halide lamps].
The challenge with the photography is that these are all essentially lumps of gray metal. 90%+ of all the elements are gray metals and shapeless. One of the few other photo periodic tables out there was published in the 1960s by Time-LIFE, and they all look the same—not very interesting! Our job was exactly like the job of a commercial photographer, an advertising photographer. They’re given a product that might be the most boring thing you ever saw, and they have to bring out its inner beauty by way of lighting and arrangement. We tried to think of the most exciting examples of each metal. We also did cheat a bit, with some of the beautiful, colorful crystals, which are not pure elements but rather compounds. But it is an excuse to put a splash of color on every page!

SPhD: You know I’m going to ask… you’re the Element King… What’s your favorite element and why?
TG: Ahh, the favorite element question! My first answer is that I don’t have a favorite child, either. But there’s different elements that I like for different reasons. Sodium, for example, if you have a lake near-by, is the element you want to have. [Editor’s note: BE CAREFUL! HIGHLY EXPLOSIVE!] On the other hand, something like titanium is a wonderful metal in every way—it doesn’t rust, it’s malleable, highly useful, strong, and many of its applications are interesting and exotic. Copper is so great as well, because it’s the only colored metal that is reasonably priced and not explosive. Cesium explodes, and gold is very expensive. That leaves copper, but people don’t tend to make sophisticated shapes out of it other than jewelry. But if I had to name two, it would be sodium and titanium.

SPhD: Let’s talk about your other book for a moment, Mad Science. Some of my favorite experiments that I want to replicate are the lightbulb, homemade pencils, 1-volt liquid battery (which I’ll use to charge up my iPod), plating the iPod, and my personal favorite, preserving a snowflake. Was there an experiment in this book that made it in or didn’t where you either felt like your life was in danger or you were like, “OK, change of plans, we’re not doing this.”?
TG: I have a policy of not doing anything dangerous. These experiments [from the book] are only dangerous if you don’t do them right, which I explain fairly well in the safety section. Because basically, I’m a complete ninny. I’m terrified of that stuff, which is the way that you ought to be. There is a good bit of thought that went into the worst case scenario, and if there was a shred of doubt, we rethought the experiment—smaller scale, or do it differently. One of the advantages of doing it for photography as opposed to in person is that we were able to get away with extremely small quantities. We also hired specialists where applicable, such as an electrical engineer for shrinking coin trick, or an industrial chemist for the chlorine experiment.
The one experiment that I suggest your readers do is the snowflake one, which is just so nice, and only took me three or four tries. The key is to keep your slide really cold and pre-cooled, and to handle them with your hands the least amount possible.
SPhD: You are, of course, most well known for your Popular Science column Gray Matter. How has writing Gray Matter, particularly focused for a lay audience, changed your role in science?
TG: It’s interesting to be faced with the challenge of trying to explain something that is actually quite complicated and deep in a format where you only have a few hundred words and you can’t assume that your audience has four semesters of college chemistry. If I were writing for Scientific American, it would be quite different and you could use a certain shorthand quite freely. Here, I have not not only explain everything, but also make it fun and entertaining. The most important thing is that I have an opportunity to speak to people, particularly young people, who probably aren’t getting any actual science anywhere else. Rather than preaching to the choir about the value and interestingness of science, I can reach out to a new audience. Often in lay magazines, it’s frustrating to read so much pseudo-science because the people doing it don’t actually know what they’re talking about. The other thing I like to do is scatter some breadcrumbs throughout my writing to leave hints or terms that can’t really be explained to encourage people to look things up on their own.
SPhD: So much of our modern culture is influenced by the growth of technology and science, which play a huge role in our lives and economy than ever before. And yet, as a whole, mainstream public knowledge of basic science can be shockingly absent or misinformed. What is the single most important thing people like you, myself and others in the science community can do to mitigate this?
TG: There’s a couple of things. One is, and I’ve written a couple of blog posts about this for Powell’s Books, that you need to be relatively fearless in getting out there and telling the truth without worrying about repercussions or consequences. I’m talking about things like creationism or homeopathy, which are two basically stupid ideas which are very widely believed. People like me have a responsibility not to beat around the bush [as to their accuracy or lack thereof]. One needs to have strong attitudes because that’s what gets people riled up. At the same time, one needs to communicate that science is something that is worth putting effort into and learning about, because it’s very powerful. If you understand it, and your competitor doesn’t, you win, in many, many situations. We see this both in technology and at the societal level, one that we’re frankly losing to China. While we’re arguing about evolution and homeopathy, they’re gathering all the natural resources they can to become the dominant global economy next Century.
SPhD: You are one of the few scientists that have crossed the barrier to entertainment and wider popular appeal. Why do you think more academics have not? Is it a problem of the public welcoming these figures, hesitation in the academic community of going mainstream, or something in the middle?
TG: It depends. There’s different motivations. I, for example, grew up in an entirely academic household; both of my parents were math professors. I completely understand that world and that mindset. The kind of articles that I write for Popular Science would be considered more than a little bit embarrassing if I were an academic and had to worry about tenure committees and citations of academic papers. It’s not a very respectable profession, from an academic point of view, and I think that keeps a lot of people out who could write for a broader popular audience. It’s also a quite different skill, one that’s taken me decades, to develop the writing style that works for a lay audience—and it’s not easy to do.
And then on the other side, there’s a resistance among the publishers and media types to take science seriously because they assume it’s going to be boring. It irritates me greatly when TV shows about science end up being frantically edited, with lots of flashy graphics going up. And you can just tell that what happened was that none of the people involved knew anything about science (or possibly cared). But if you look at a show like MythBusters, for example, it’s a fantastic science show.
SPhD: Yes! We love MythBusters!
TG: They are one of the most successful of all cable shows, and they are 95% a science show wrapped up around the theme of a “myth” that they have to “bust.” Fundamentally, what they do is pose a question and then hypothesize and answer it using the scientific method. I don’t know if people appreciate how revolutionary it is for them to do that consistently.
SPhD: This is why ScriptPhD.com loves The Discovery Channel and MythBusters so much. We are in contact with them, we’re big supporters, and we wish them, and you, a lot of luck with shepherding the next generation of curious young scientists!
TG: Delightful to talk to you as well. Thanks!
~*ScriptPhD*~
*****************
ScriptPhD.com covers science and technology in entertainment, media and advertising. Hire our consulting company for creative content development.
Follow us on Twitter and our Facebook fan page. Subscribe to free email notifications of new posts on our home page.
]]> I must preface this next post with a little truth in advertising. I’m a chemist. True blue, to my very core. College degree in physical chemistry, PhD in chemistry. So when I heard about a cable show on AMC whose whole premise rested on a chemistry teacher manufacturing meth, I must say, I was slightly skeptical. The propensity for letdown was huge, both in plot and in science. Well, let me assure you that Breaking Bad broke good. A “Break”out hit in its second season, the show has managed to layer complex serialized storytelling with compelling characters and stories, and even better science. In fact, chemistry itself can very well be considered a recurring character on this show and we’ll highlight some of the best moments in a bit.
I must preface this next post with a little truth in advertising. I’m a chemist. True blue, to my very core. College degree in physical chemistry, PhD in chemistry. So when I heard about a cable show on AMC whose whole premise rested on a chemistry teacher manufacturing meth, I must say, I was slightly skeptical. The propensity for letdown was huge, both in plot and in science. Well, let me assure you that Breaking Bad broke good. A “Break”out hit in its second season, the show has managed to layer complex serialized storytelling with compelling characters and stories, and even better science. In fact, chemistry itself can very well be considered a recurring character on this show and we’ll highlight some of the best moments in a bit.
ScriptPhD Grade: A+
The Premise
If the pilot episode doesn’t get your attention in the first five minutes, then I don’t know what will. A man wearing nothing but his skivvies and a gas mask careens a Winnebago in the New Mexico desert, a passed out body beside him, two more dead in the back, and a toxic sludge of chemicals seeping on the floor. With impending sirens approaching, he videotapes a final goodbye and apology to his family. Through flashbacks, we come to find out that the man is Walter White, an unassuming chemistry teacher in Albuquerque, NM. While on his humiliating moonlighting shift as a car wash attendant, because we pay our public school teachers so well, Walt collapses. The culprit? Lung cancer. Terminal. Inoperable. He decides to infuse some excitement into his life on a bust ride with his brother-in-law, a DEA agent. Only instead of discouraging Walt, the bust shows him how much money can be made. While pondering the possibility of leaving his family financially secure after his passing, he spots an old flunky student, Jesse Pinkman, fleeing the scene. “You know the business, I know the chemistry,” he proposes to Jesse. An idea is born, and the metamorphosis of Walter White begins. Back to the original scene, the sirens turn out to be fire trucks, one of the many hair-raising escapes to come, and Walt and Jesse live to sell meth another day.
In addition to Walt (played by the talented Bryan Cranston), and Jesse (dazzling newcomer Aaron Paul), we meet Skyler (Anna Gunn), Walt’s supportive but perplexed wife, who grows to be very suspicious of him as he has a harder time curtailing his clandestine activities, and Walt, Jr., a teenager with Cerebral Palsy, sensitively portrayed by RJ Mitte. The relationships serve as a centerpiece of the show are unraveled like the plot, in layers and tantalizingly. As Walt’s own family unit faces turmoil, Jesse, too, is disowned by his for his drug use. What started out as a business transaction between a teacher and former student blossoms into a tender father-son relationship. Meanwhile, while Walt’s well-meaning DEA brother-in-law Hank (Dean Norris) closes in on the hottest new meth dealer in town, Walt and Jesse face a series of personal and professional setbacks. For every two steps forward, for every dollar made, there is a new foe, a new nemesis, or new unintended collateral. All of the action culminates in an electrifying Season 2 finale sure to generate buzz and anticipation for Season 3.
The Science
Science on Breaking Bad is given the red carpet treatment: it’s sleek, sexy, geek-chic, tongue-in-cheek and everywhere. The show revels in delightful touches such as the title credits interspersing elements from the periodic table. Walt’s classes brim with interesting blink-or-you-miss-it factoids, such as H. Tracy Hall inventing the first reproducible process for making diamonds. To a stupefied, gun-happy Jesse, he makes the suggestion of killing a drug lord with castor beans, the source of the protein toxin ricin. And let’s not mention the two separate synthetic methods he comes up with to cook and crystallize the best meth the New Mexico DEA has ever seen. The darkly comedic highlights of the show are Walt and Jesse’s interactions in their “laboratory”, a beaten-down Winnebago camper. Shocked by Jesse’s sloppy street cooking, Walt pilfers glassware and equipment from his classroom—gas masks, round bottom flasks, reflux condensers, crystallization dishes—to build a setup worthy of Pfizer. Along the way, Jesse gets some remedial chemistry that he failed back in high school. I mean, sure, they’re making a devastating and highly illegal narcotic, but at least it’s via a proper Grignard reagent amination of a Schiff base!
On a more serious note, Breaking Bad also strives for a VERY candid and unrelenting portrayal of both cancer and the ramifications of the modern-day drug trade. Often whitewashed in entertainment, Walt’s cancer, and the side effects are shown in a brutal way, but the stark realism also underscores his desperation as the illness unfolds. Easily on par with David Simon’s brilliant The Wire on HBO, in the world of Breaking Bad no one is absolved from the intertwining effects of drugs—the rising body count, both from use and dealing, the strain on law enforcement, and families torn apart. In an astute opening TRULY ripped from the headlines, a Season 2 Breaking Bad episode starts with an original narcocorrido, a Mexican drug ballad evolved from its folk music tradition that is often used to chronicle the drug trade and escalating violence over the last two decades. Take a look:
Bottom line: the science is white-hot, the writing is red-hot, the meth is blue and the humor is black, so why aren’t you watching?
Accolades
Breaking Bad has been the recipient of a number of recent awards and critical acclaim. They won a 2009 Peabody Award for excellence in television achievement. Bryan Cranston won the 2008 Emmy for Outstanding Leading Actor in a Dramatic Series. Series creator and executive producer Vince Gilligan won a Writers Guild of America award for the Pilot episode. Many more achievements are sure to come for their outstanding sophomore effort!
For the ScriptPhD.com Top 4 Walter White Chemistry Moments in the show thus far and an in-depth discussion of the neat science behind them, click “continue reading”…
Top 4 Walter White Chemistry Moments
4) A salt and battery…
Sometimes, desperate situations call for desperate (and clever!) chemical measures. The sticky wicket? Jesse, as you will come to learn when you watch this show, is not the sharpest knife in the drawer. While on an exotic synthetic staycation in the lovely New Mexico desert, he stores the key to his Winnebago-cum-meth lab in the ignition. Two days later, the result is a dead battery and no one around for miles to help. D’oh! To make matters worse, their spare generator catches on fire, they run out of cell phone charge, and the only person who knows how to come get them gets lost. Walt suggests rebuilding a battery.
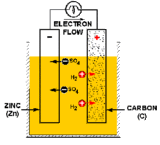
A battery (or a voltaic cell) is really just a series of fuel cells that store combined chemical energy to provide a high source of voltage power. And a fuel cell is an electrochemical device, or a galvanic cell (named after its inventor, Luigi Galvani), which converts free energy of a chemical reaction into electrical energy, or electricity. Generate electricity, generate charge, find a way to collect said charge, you’ve got power. Simple, right? In the case of batteries, the basic building block is a primary cell (also called a simple galvanic cell), which is made of four components: an anode electrode (the negative end), a cathode electrode (the positive end), an electrolyte solution that will generate positive and negatively charged ions for the two chemical reactions that take place, and a conductor (usually a wire) to carry the current of the electrons from one side to the other. The flow of charge ALWAYS goes from the cathode (the positive end) to the anode (the negative end).

Now that we are armed with the basics, here’s how Walt built his galvanic cells. For the anode (or source of negative charge) he uses a galvanized metal, specifically zinc, which they get by collecting coins and spare metallic parts (nuts, bolts, washers, etc). For the cathode (the positive charge where the current will flow out), Walt uses graphite and mercuric oxide that he ground down from the Winnebago’s brake pads. For the electrolyte, he soaks a sponge in potassium hydroxide (KOH). So in this case, referring back to the picture of the galvanic cell, the flow of positive charge will come in the form of K+ ions, and the negative charge from the OH– ions. Remember, we also need a conductor to carry the current of electrons—the charge—from one half-cell to the other. Walt uses copper wire, which he then connects to the jumper cables by collecting all six cells and pooling the electric current to restart the van’s battery.
Realistically speaking would this actually restart the camper? Sadly, probably not. We’ve talked about what a galvanic cell is, now let’s talk about how it works. The charge, or electric current, is generated by two separate chemical reactions (or half-reactions) that occur on either electrode of the cell. At the anode, an oxidization reaction strips electrons from the electrode (usually a metal of some sort), resulting in overall negative charge. At the cathode, free electrons that have traveled through the conducting circuit are used to reduce the electrode species to generate a positive charge. Taken together, these two values add up to the total cell potential, defined as the ability to force electrons through a circuit, and measured in voltage.
The zinc-carbon cell that Walt has built is a variation of a classic Bunsen cell, and we can estimate the cell voltage at around 2 total Volts. If we break down the two half-reactions, oxidation and reduction, we can add up the total potential using a fancy-schmancy mathematical equation called the Nernst equation. The potential of the mercuric oxide reduction, used in commercially available mercuric oxide batteries, is 1.35 Volts, and the potential of zinc oxidization is -0.76 Volts. To determine the full potential of a cell, we subtract the anode from the cathode, and this reaction adds up to 2.1 Volts. Walt mentions that he only has material enough for six cells. So if we add this up, we get enough voltage, give or take, to build a typical 12 Volt battery. Perfect in theory. But, when we talk about real-life circuits, we have to factor in electrical resistance, the opposition of electric current. Resistance occurs in all sorts of conductors, including metals (due to electron scattering) and ionic liquids (depending on concentration and insularity of the solution medium). If we’re going to get super-technical, we could invoke a physics property called Ohm’s Law which states that the ultimate current (which we measure in amperes) potential is inversely proportional to its resistance. The higher the resistance, the lower the ultimate current. So while Walt was able to build a battery with the proper voltage, the internal resistance would likely not generate enough current to provide the immense power necessary to jump-start a car that big. Most cars use a standard 12 Volt battery, but for cold-cracking need about 400-600 Amps, higher if you are going to start something like the Winnebago. The battery that Walt built would probably generate 20-30 Amps of current, based on current for a typical basic Galvanic cell, and that’s being generous. But how many shows on the air are even attempting this kind of clever science? So Breaking Bad gets an A for effort and that’s just all there is to it!
Incidentally, you can easily make your own galvanic cell at home. Check out this neat video of six lemons powering a low-wattage LED bulb:
Lemon Battery – Watch more funny videos here
3) What’s your name?
Of course, every drug kingpin worth his weight in meth has to have a nom de guerre. Mr. White’s sobriquet choice? Heisenberg. I must admit my inner chemistry geek did major cartwheels when I heard this. It’s such an appropriate name for him on so many levels. Heisenberg, of course, is a tongue-in-cheek reference to Werner Heisenberg, one of the great physicists of our time. He is the father of the “Heisenberg Uncertainty Principle” in quantum mechanics, which states that measured values for a particle’s position and momentum cannot be ascertained simultaneously with equal precision. It turns out that particles actually act more like waves, whose three-dimensional spatial function can be separated into three axes, or directions, x, y and z, horizontal, vertical and diagonal, respectively. So, if for a certain particle we can specify momentum with absolute certainty, then its position can be found anywhere along those axes with an equal probability. For this contribution, Werner won a Nobel Prize in physics in 1932.
What a clever and ironic name for Walt, whose own life and identity faces so much uncertainty as the show unfolds: in his cancer diagnosis, his long-term prognosis, his deteriorating relationship with his family, his precarious one with Jesse, and the theoretical uncertainty of his motivations for manufacturing meth, which certainly evolve over the two first seasons.
It’s important to note Heisenberg is also a very controversial historical figure. He played a prominent role in the German nuclear energy project, the race to develop an atomic bomb prior to World War II, although never formally affiliated with the Nazi movement. He later joined a prominent group of scientists who opposed the use of tactical nuclear weapons as warfare. The Tony-award winning play “Copenhagen” centered around the possibilities of a mysterious meeting in 1941 between Heisenberg and fellow physicist Neils Bohr in German-occupied Copenhagen around which there remains to this day… well… uncertainty.
A big ScriptPhD props to the writing team on Breaking Bad for this little gem!
2) It’s enough to just melt you!
Not since the CSI Season 2 episode “Bully For You”, in which a victim’s body found in a duffel bag decomposed so badly it had to be poured out, has a liquefied body played such an important role on a television episode. You’ve just killed the drug dealer who was supposed to peddle your batch of crystal meth, but you have to make the body disappear quietly without your DEA brother-in-law finding out. I mean seriously, folks, we’ve all been there, right? “The best thing to do,” Walt concludes, “is dissolving [the body] in strong acid.” We’ll talk more about this in a second, but first, check out the minisode for the episode “The Cat’s in the Bag…”. Pay particular attention to the disastrous results of this suggestion in the last 1:30, especially if you have a strong stomach.
In the episode, Walt asks Jesse to pick up a plastic container in which to dissolve the body. Even Jesse, dubious of this, says to him, “Any decent acid is gonna eat right through this.” “Not hydrofluoric [acid],” Walt concludes. What? An acid that can eat through flesh and bones but will leave a flimsy plastic bucket intact? This sounds like crazy talk! Well, actually, he’s right. An acid, chemically speaking, is nothing more than a compound that is able to give up a proton to a willing base in solution. By proton we mean a positively-charged hydrogen (H+) with water acting as the accepting base in most cases. The stronger the acid, the more readily it gives up this proton, and the higher the concentration of H3O+ protonated water ions floating in solution, called the dissociated state. Why wouldn’t it react with plastic? Walt tells Jesse to look at the bottom of the bins for a symbol called “LDPE”. He’s talking about low-density polyethylene, the plastic polymer that makes up everything from plastic bags, various containers, dispensing bottles, wash bottles, tubing, to molded laboratory equipment. The repeating units of CH2, some linked by side branches, make for a strong, compact bond and very low reactivity. That is, this chemical will neither give up its hydrogens when exposed to a solution, nor will it accept any. In this case, the acid will primarily disintegrate the body, which as we know is about 80% water, thus creating a solution, but the nonreactive hydrocarbon of the bin would remain chemically inert.
Hydrofluoric acid, contrary to Walt’s statement, is NOT really that strong of an acid, compared to other options, but has some interesting properties useful for Walt and Jesse’s predicament. Back up for a moment. There’s two ultimate determinants of acid strength—electronegativity (the charge on the molecule that the hydrogen is attached to) and the size of the atom that the hydrogen is attached to, which has to do with the strength of the bond. As you move across the periodic table, atom charge strongly increases, which is why fluorine is a much better acid candidate than nitrogen or an unreactive atom like carbon. BUT, as you move down the periodic table, elements get bigger and bigger, and as their size increases the strength of the bond with hydrogen weakens for various reasons. (We’ll skip discussion of covalent bond orbitals for next time!) So acidically speaking, HF < HCl (stomach acid) < HBr < HI. Personally, I really would have chosen a very concentrated hydrochloric acid or sulfuric acid solution like John George “The Acid Murderer” Haigh, or better yet, liquid lye (the base NaOH), which would be able to react with the organic components of tissue and fat content and wouldn’t have dissolved Jesse’s tub, but where’s the drama in that? Realistically speaking, HF would not be able to liquefy the body to the substantial extent shown in the episode. But because it is a weak acid and exists primarily in its undissociated state, it is able to penetrate deeply into the skin before deprtotonating, thus making it an excellent and efficient corrosive for human flesh (and very dangerous!). That’s right, it eats your body from the inside out. More importantly, it reacts strongly with calcium and magnesium, so it would be able to efficiently dissolve Emilio’s bones for disposal. [Incidentally, when people are treated at the hospital for HF poisoning, the first line of treatment is calcium gluconate to “compete” with the calcium in your bones as a neutralization reaction.] Unfortunately, it also reacts with silicon dioxide, the major component of glass and ceramics. Hence the look of absolute and profound horror on Walt’s face as Liquid Emilio, the bathtub, and everything in-between come crashing down.
Take home lesson? If you’re desperate to get rid of a dead body by chemical disincorporation, for God’s sakes, read up on your chemistry first!
Annnnnnd ::drumroll:: the best Walter White chemistry moment to date?
1) A little tweak of chemistry!


Without question, the scientific highlight of the show to me, thus far, is the integration of clever reaction chemistry to advance a major plot on the show. Early in the episode “Crazy Handful of Nothin’”, Mr. White is teaching his class about the power of chemical reactivity. Sometimes, it’s gradual and imperceptible, to which he gives the example of metal oxidation. But other times, the reaction is violent, quick, and produces tons of energy. On the chalkboard, he writes an example of an explosive compound, mercury fulminate, Hg(ONC)2. Relatively easy to make synthetically, fulminated mercury is a powerful explosive and was long used as a detonation primer for dynamite.
This chemistry lesson proves to be a dynamite primer of its own later in the episode. The first good large batch of meth—1 pound!—that he and Jesse were able to synthesize was stolen without compensation by Albuquerque’s toughest drug wholesaler. What’s a wronged high school chemistry teacher to do? Walt shows up at Tuco’s casa with a large crystal of what looks like more meth and a demand for his money. But Walt surprises him by announcing that it isn’t in fact meth, and throws the crystal to the ground. The result? KABOOM! See the before and after pictures. What is that stuff, a stunned Tuco asks. “A little tweak of chemistry.” Most notable about this scene is that it caps the season-long transformation of Walt’s character. As he walks over to his car, money in hand, reputation restored, he hasn’t just earned the respect of a thug. This is the moment in the show that delineated a before and an after, where the line was drawn in the sand, and we never again saw the innocent sick chemistry teacher desperate to save his family. A drug dealer was born.

The only slight criticism that I have is that in reality, mercury fulminate wouldn’t actually look like the large meth-like crystals portrayed on the show. The chemical synthesis process usually leads to very fine white powder precipitate crystals like the one in the picture on the right. But we can just assume that Walt used his past career as a “Crystallographer Extraordinaire” to produce the largest mercury fulminate crystals to date ;-). Secondly, this stuff is unstable and extremely reactive. As in don’t touch it, don’t expose it to light, don’t mess with it unstable. It can be detonated by sparks, shock, friction, or even a wayward glance. Realistically speaking, Tuco handling the stuff with his knife and dropping it on the table would be enough to ignite it. We’ll also have to assume Walt was very careful in handling an entire plastic bag of it. Nevertheless, this whole scene is so creative, well-written and downright badass, that here at ScriptPhD.com, we can’t allow minor quibbles to detract from the moment.
The season finale of Breaking Bad aired May 31, 2009 on AMC, but for those of you not yet watching this spectacular series, there is plenty of time to catch up. The Season 1 DVD is available in stores and the Season 2 DVD release date will be updated on our site as soon as we know it! And we’re going to do our darndest to welcome Vince Gilligan, the show’s brilliant creator and executive producer, to our in-house ScriptPhD lab to chat all things Breaking Bad! Stay tuned!
For a look ahead at Season 3, check out this sneak peek preview:
All video clips and pictures are © 2007-2009 AMC Television and Sony Pictures Television. All rights reserved.
~*ScriptPhD*~
]]>
