
Last year around this time, ScriptPhD.com posted Breaking Bad, Chemistry Good, an in-depth article about AMC’s breakout hit Breaking Bad, and its stunningly accurate science content. Walter White, the show’s anti-hero, is a cancer-stricken high school chemistry teacher who starts cooking and dealing methamphetamine for financial security. In our article, we highlighted several clever uses of chemistry throughout the show’s run that not only integrated brilliantly into the plot but had realistic real-world applications as well. What a difference a year makes! Last week, Editor Jovana Grbić sat down with Breaking Bad‘s delightful Creator and Executive Producer Vince Gilligan to talk about the show’s origins, the science, and some behind-the-scenes secrets that will surprise even dedicated fans. We hope you enjoy reading our interview as much as we enjoyed chatting with him. The secrets of Breaking Bad, under the “continued reading” cut.
ScriptPhD.com: As a scientist (and PhD chemist in particular), Breaking Bad’s premise was very intriguing to me from the start. Can you share a bit about the inspiration for its origins?
Vince Gilligan: You know, Jovana, I wish I always had a better answer for where it all comes from. But I only can put a date to things—I only seem to remember when ideas came to me. In this case, Breaking Bad was an idea that came to me when I was speaking to an old college friend on the phone. He and I are both writers, and were both bemoaning the fact that—this was was about 2005—we were having trouble getting work and we were wondering where our next writing gig was going to come from. We thought instead, maybe we should switch occupations. Maybe we should buy an old RV and put a meth lab in the back and ride around in the Southwest. And we were joking around, but this character that became Walter White jumped into my head very quickly, right in the midst of this phone conversation. That’s when the idea came to me!

I have to say, as far as the science angle goes, I’ve always loved science, and yet I never had any real facility for it. I’ve always been a terrible student of mathematics in high school and in college. I’ve never even taken chemistry, I hate to admit it. But I’ve always loved science. And for most of my life, I’ve been a subscriber of Popular Science magazine and Popular Mechanics. But I’ve never made the jump from Popular Science to Scientific American! I don’t have a deeper understanding, but I love the idea that there are real concrete black-and-white answers in science and math, that we don’t unfortunately get in the rest of our life. The world, and our lives, is full of gray areas and uncertainties and opinion versus fact. And yet in mathematics, 2+2=4 and always has and always will. In science, certain chemicals put together in a certain way always create the same compound. I love that idea about science and I always have. I just wished in my heart of hearts that I had a deeper facility and understanding for it, but I have to say it is fun to live it by proxy and to write about a man who has a very profound understanding of chemistry and of science.
SPhD: I find it ironic hearing you say that, because a lot of people who are far less humble about their scientific capacity, their shows are far less accurate to incorporate it. Breaking Bad is one of the few shows or films that we have given a top-notch grade to for scientific accuracy and plausibility, allowing for some Hollywood liberties, of course. Who are some of the people that you consult with to write the chemistry experiments and tidbits, and what has been the general reaction within the science community?
VG: We have a lot of really good help. We have a woman named Dr. Donna Nelson, and she is the Associate Professor of Chemistry at the University of Oklahoma. When Breaking Bad first went on the air, she contacted our office pretty early on (a few episodes in) and she said she liked what we were doing and if she could ever be of any help to us, she’d be happy to be a resource. And we have taken her up on that offer, and she has been a resource to us over the seasons. We run certain moments that happen in the story by her is as accurate as possible, and she’s been a great help. I know Bryan Cranston [Walter White], our star, shadowed a UCLA chemistry professor for a week or two before he shot the pilot, to immerse himself in the world of chemistry, not just to learn a bit about chemistry, but to see what a prototypical chemistry professor looks and sounds and acts like. Also, a part of my original inspiration, to be fair, was my long-time girlfriend Holly, her brother Hank, who I borrowed the name for Walt’s brother-in-law from, got a PhD in chemistry while he was in the Navy and is a chemist for a government organization out East and is studying red tides off the Atlantic coast. I also cannot leave out a man named Dr. Victor Bravenec, and he’s a DEA chemist based out of Dallas. I have to give great credit to the DEA, because they have been helpful since Day 1 on our Pilot, and Dr. Bravenec in particular has been helpful with the chemistry of methamphetamine and the particulars of how it is created in a laboratory. I’m so proud of the fact that you’re reacting to how realistic the chemistry is on our show, and so much of that is due to our wonderful advisors.
SPhD: One of the things I love most about Breaking Bad is that chemistry acts like an integral character, one that plays a very important role in Walt’s identity, the cooking and production of the meth, and in some cases, their very survival in some pretty hairy situations (as in the case of the fulminated mercury and homemade battery). Was it always the plan to have it be such a big part of the show or is that something that evolved?

VG: I did always like the idea that this would be a cable TV version of MacGyver. That was not first and foremost when I was coming up with the idea. First and foremost to me, the show is a character study about a man who is undergoing a radical transformation. He’s transforming himself from a protagonist to an antagonist. The whole show, in that sense, is an experiment to continue chemistry analogy. But there was always an element that I thought we could have fun with is the MacGyver aspect, as it were and the idea of using [Walt’s] knowledge to get him out of a jam every now and then. And I have to confess, as much fun as we have with that stuff, the show has taken so many dark character turns as he progresses from a good guy into a bad guy that of it goes by the wayside, although not intentionally. But those moments you speak of were a lot of fun to come up with and a lot of fun to write, and I’m hoping we’ll find a way to insert more of those moments as the season progresses.
SPhD: You’ve brought it up, so let’s just talk about where Breaking Bad goes from here. To me, Season 1 was very much about the construction of these characters, their situation, the “building up” of their individual new worlds. In many ways, Season 2 was the de-construction, where each character felt something fall apart in some way; Walt’s lies and second life, Jesse’s addiction and girlfriend’s death, Skyler’s trust in Walt, and possibly Hank’s DEA career. What do we look forward to in Season 3?
VG: Good question. And I hate to admit it, but I’m not the best at looking forward. You know the old expression “You can’t see the forest from the trees?” We are so deep into these characters’ lives that sometimes, I myself and my writers are not the best people to ask for a complete bigger picture explanation of what in fact is going on. All I know for sure is that Walt is in the process of change and transformation, and it’s an interesting experiment, one that we’re doing for the first time, as opposed to a repeated experiment. We can’t even predict the outcome!
SPhD: What’s funny is seeing the first few episodes of Season 3, I get the feeling Walt misses the badness and the danger of it all. He wants so badly to be good and to repudiate this person he’s become, and yet, I get the feeling he misses some of the perks and the thrills that it brought. Am I completely off-base here?
VG: No you are not off-base at all. That’s very astute. He’s an interesting character, because a lot of what this show is about to me is the human capacity for rationalization. We all rationalize our behavior to ourselves, our ideas, our actions. It’s just something that human beings do, it’s part of us. We usually do it in small, minor, insignificant ways. But Walt takes that to the nth degree. He stretches that to the breaking point. He’ll go around saying to himself, and when pressed by others to them, that this criminal behavior he finds himself engaged in is done purely to aid his family. He does it for their financial benefit. And yet we put the light on that pretty early in Season 1. If you’ll remember, he gets this offer from his rich former lab partner for a great job that sounds like it’s no strings attached and with all the money that he’ll need to treat his cancer and for his family to be financially solvent. And lo and behold, he doesn’t take it. That’s one of the moments I’m most proud of on our show, because prior to that moment, the show could have gone in a very different direction—we could have had the show become a weekly procedural. This week the meth lab burns down, and next week his money gets eaten by rats, and the next week after that the police arrest him, but he has to weasel out of that.
But early on we all recognized that a show like this can’t reach its full potential if we don’t really examine this guy at a deeper level. A lot of people, millions of people, especially with the economy being in the toilet, face similar situations, unfortunately. People face the issues that Walt faces, where money is a real problem. And most people don’t decide to cook crystal meth! So we realized early on that Walt has to have some kind of darker component to him that he’s perhaps always had. This is a man who is, above all else, prideful. And he bursts with pride at this avocation that he’s taken up, and he’s prideful about his product, about its quality. In so many ways, he’s just a sad little man who has felt passed over his whole life. He feels like he hasn’t really existed until he’s become a criminal, until he’s broken bad.
SPhD: My favorite episode to date has been the Season 2’s Peek-a-Boo, and its exploration of the beautifully complex character of Jesse Pinkman. His filial relationship with Walt is a cornerstone of this show, and has rather ironically blossomed amid the self-destruction of Walt’s relationship with his family and Jesse’s estrangement from his own parents. Can you talk about their relationship and your take on how these two individuals fit in each other’s worlds?
VG: Absolutely! It is a very interesting relationship, and it’s one that I didn’t realize what it would become. I’d love to say I knew from Day 1 when I was writing the pilot what this show would be, exactly, and all its dynamics, but the truth is that they’ve been a work in progress. A lot of this grows and creates itself, and the actors and writers bring so much to it, and it’s amazing to stand back years later and realize what you’ve had a hand in creating. But yes, it’s very much a father-son relationship between Walt and Jesse. And I have to say, as further evidence that this wasn’t the original intention of the show, my intention was to kill Jesse off at the end of Season 1. He was going to be kind of a character who helped Walt get into the business, and then got violently killed in a drug deal gone wrong. And the whole point of his existence would have been to get Walt into the business and then give Walt a reason to feel very, very guilty, further fueling bad behavior on his part. Luckily, we hired Aaron Paul to play Jesse, and he is such a fine young actor and so talented and charismatic that very quickly on, like in Episode 1 or 2, I realized that there was no way I was killing him off.
SPhD: And where do they go from here? They’re in a tenuous place right now. Together but apart, not really sure where things are going.
VG: It’s interesting, you put your finger on quite a lot of it. And it’s very much a love-hate relationship between these two guys, but it’s mostly love-hate on the part of Jesse. There are times when I’m not sure Walt has any regard for Jesse at all. Very often, there’s a coldness to Walt, and a desire just to get the job done and he sees Jesse as a useful tool but not much else. He doesn’t seem to have much regard or respect for Jesse, so every now and then, when he throws Jesse a scrap of respect, you feel how thirsty Jesse is for that. It’s funny, Jesse is sort of the moral center of our show. Even though he started off in the business before Walt, and Walt was a straight-arrow character before we meet Jesse, somehow Jesse seems to have a more refined and defined moral center. Very often, Jesse is the one who says “Should we really be doing this? Aren’t we being greedy? Haven’t we made enough money already?” He really is the moral center of the meth trade that we portray on the show, and Walt, who should be a father figure to him, who should be ushering Jesse out of the business, and a large part of us as the audience wishes that he would do the right thing. We know in our hearts that he most likely never will.
SPhD: They’re all beautiful relationships and I can’t wait to see where they all go. I love that you’ve even made me hate Skylar [Walt’s long-suffering wife], which I never thought I would do. I’m so sorry for that!
VG: It’s so funny, and I’m glad you brought that up, because you are definitely not even close to being the first person to say that. It’s just a funny thing about storytelling. Even when your hero is not a nice person, and he’s doing bad things, because he’s your protagonist, if you’re along for the ride, then you start to see the world through his eyes. It’s the nature of storytelling. It’s the same thing when you’re watching The Sopranos or some other show where there’s someone who is pretty much a reprehensible character is nonetheless your protagonist. You’ll root for that person to succeed. And right now, Skylar gets in the way, on a purely mechanical level, of Walt’s success and happiness and therefore we see her as an obstacle and we don’t like her for what she’s doing. So you’re not alone in feeling that way. But the funny thing is, I see Skylar as the good guy and Walt as the bad guy. I love Walt! He’s a great guy to write for, but he’s kind of a monster when you think about it. He shouldn’t be breaking back into the house, trying to get back into her good graces when the things he’s done and the lies he’s told really make him not the good guy. She’s being heroic when she doesn’t tell the police on him.
SPhD: Thanks, Vince, and we really appreciate you joining us!
VG: Thanks, and it really makes me feel good to hear that you think the science is authentic, because we do our best, and we will continue to.
Interested in watching the show? Start with this brilliant six-minute catch-up video:
Season 3 episodes of Breaking Bad air Sundays at 10 PM on AMC.
~*ScriptPhD*~
*****************
ScriptPhD.com covers science and technology in entertainment, media and advertising. Hire our consulting company for creative content development.
Follow us on Twitter and our Facebook fan page. Subscribe to free email notifications of new posts on our home page.
]]> I must preface this next post with a little truth in advertising. I’m a chemist. True blue, to my very core. College degree in physical chemistry, PhD in chemistry. So when I heard about a cable show on AMC whose whole premise rested on a chemistry teacher manufacturing meth, I must say, I was slightly skeptical. The propensity for letdown was huge, both in plot and in science. Well, let me assure you that Breaking Bad broke good. A “Break”out hit in its second season, the show has managed to layer complex serialized storytelling with compelling characters and stories, and even better science. In fact, chemistry itself can very well be considered a recurring character on this show and we’ll highlight some of the best moments in a bit.
I must preface this next post with a little truth in advertising. I’m a chemist. True blue, to my very core. College degree in physical chemistry, PhD in chemistry. So when I heard about a cable show on AMC whose whole premise rested on a chemistry teacher manufacturing meth, I must say, I was slightly skeptical. The propensity for letdown was huge, both in plot and in science. Well, let me assure you that Breaking Bad broke good. A “Break”out hit in its second season, the show has managed to layer complex serialized storytelling with compelling characters and stories, and even better science. In fact, chemistry itself can very well be considered a recurring character on this show and we’ll highlight some of the best moments in a bit.
ScriptPhD Grade: A+
The Premise
If the pilot episode doesn’t get your attention in the first five minutes, then I don’t know what will. A man wearing nothing but his skivvies and a gas mask careens a Winnebago in the New Mexico desert, a passed out body beside him, two more dead in the back, and a toxic sludge of chemicals seeping on the floor. With impending sirens approaching, he videotapes a final goodbye and apology to his family. Through flashbacks, we come to find out that the man is Walter White, an unassuming chemistry teacher in Albuquerque, NM. While on his humiliating moonlighting shift as a car wash attendant, because we pay our public school teachers so well, Walt collapses. The culprit? Lung cancer. Terminal. Inoperable. He decides to infuse some excitement into his life on a bust ride with his brother-in-law, a DEA agent. Only instead of discouraging Walt, the bust shows him how much money can be made. While pondering the possibility of leaving his family financially secure after his passing, he spots an old flunky student, Jesse Pinkman, fleeing the scene. “You know the business, I know the chemistry,” he proposes to Jesse. An idea is born, and the metamorphosis of Walter White begins. Back to the original scene, the sirens turn out to be fire trucks, one of the many hair-raising escapes to come, and Walt and Jesse live to sell meth another day.
In addition to Walt (played by the talented Bryan Cranston), and Jesse (dazzling newcomer Aaron Paul), we meet Skyler (Anna Gunn), Walt’s supportive but perplexed wife, who grows to be very suspicious of him as he has a harder time curtailing his clandestine activities, and Walt, Jr., a teenager with Cerebral Palsy, sensitively portrayed by RJ Mitte. The relationships serve as a centerpiece of the show are unraveled like the plot, in layers and tantalizingly. As Walt’s own family unit faces turmoil, Jesse, too, is disowned by his for his drug use. What started out as a business transaction between a teacher and former student blossoms into a tender father-son relationship. Meanwhile, while Walt’s well-meaning DEA brother-in-law Hank (Dean Norris) closes in on the hottest new meth dealer in town, Walt and Jesse face a series of personal and professional setbacks. For every two steps forward, for every dollar made, there is a new foe, a new nemesis, or new unintended collateral. All of the action culminates in an electrifying Season 2 finale sure to generate buzz and anticipation for Season 3.
The Science
Science on Breaking Bad is given the red carpet treatment: it’s sleek, sexy, geek-chic, tongue-in-cheek and everywhere. The show revels in delightful touches such as the title credits interspersing elements from the periodic table. Walt’s classes brim with interesting blink-or-you-miss-it factoids, such as H. Tracy Hall inventing the first reproducible process for making diamonds. To a stupefied, gun-happy Jesse, he makes the suggestion of killing a drug lord with castor beans, the source of the protein toxin ricin. And let’s not mention the two separate synthetic methods he comes up with to cook and crystallize the best meth the New Mexico DEA has ever seen. The darkly comedic highlights of the show are Walt and Jesse’s interactions in their “laboratory”, a beaten-down Winnebago camper. Shocked by Jesse’s sloppy street cooking, Walt pilfers glassware and equipment from his classroom—gas masks, round bottom flasks, reflux condensers, crystallization dishes—to build a setup worthy of Pfizer. Along the way, Jesse gets some remedial chemistry that he failed back in high school. I mean, sure, they’re making a devastating and highly illegal narcotic, but at least it’s via a proper Grignard reagent amination of a Schiff base!
On a more serious note, Breaking Bad also strives for a VERY candid and unrelenting portrayal of both cancer and the ramifications of the modern-day drug trade. Often whitewashed in entertainment, Walt’s cancer, and the side effects are shown in a brutal way, but the stark realism also underscores his desperation as the illness unfolds. Easily on par with David Simon’s brilliant The Wire on HBO, in the world of Breaking Bad no one is absolved from the intertwining effects of drugs—the rising body count, both from use and dealing, the strain on law enforcement, and families torn apart. In an astute opening TRULY ripped from the headlines, a Season 2 Breaking Bad episode starts with an original narcocorrido, a Mexican drug ballad evolved from its folk music tradition that is often used to chronicle the drug trade and escalating violence over the last two decades. Take a look:
Bottom line: the science is white-hot, the writing is red-hot, the meth is blue and the humor is black, so why aren’t you watching?
Accolades
Breaking Bad has been the recipient of a number of recent awards and critical acclaim. They won a 2009 Peabody Award for excellence in television achievement. Bryan Cranston won the 2008 Emmy for Outstanding Leading Actor in a Dramatic Series. Series creator and executive producer Vince Gilligan won a Writers Guild of America award for the Pilot episode. Many more achievements are sure to come for their outstanding sophomore effort!
For the ScriptPhD.com Top 4 Walter White Chemistry Moments in the show thus far and an in-depth discussion of the neat science behind them, click “continue reading”…
Top 4 Walter White Chemistry Moments
4) A salt and battery…
Sometimes, desperate situations call for desperate (and clever!) chemical measures. The sticky wicket? Jesse, as you will come to learn when you watch this show, is not the sharpest knife in the drawer. While on an exotic synthetic staycation in the lovely New Mexico desert, he stores the key to his Winnebago-cum-meth lab in the ignition. Two days later, the result is a dead battery and no one around for miles to help. D’oh! To make matters worse, their spare generator catches on fire, they run out of cell phone charge, and the only person who knows how to come get them gets lost. Walt suggests rebuilding a battery.
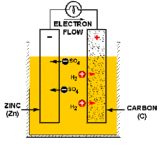
A battery (or a voltaic cell) is really just a series of fuel cells that store combined chemical energy to provide a high source of voltage power. And a fuel cell is an electrochemical device, or a galvanic cell (named after its inventor, Luigi Galvani), which converts free energy of a chemical reaction into electrical energy, or electricity. Generate electricity, generate charge, find a way to collect said charge, you’ve got power. Simple, right? In the case of batteries, the basic building block is a primary cell (also called a simple galvanic cell), which is made of four components: an anode electrode (the negative end), a cathode electrode (the positive end), an electrolyte solution that will generate positive and negatively charged ions for the two chemical reactions that take place, and a conductor (usually a wire) to carry the current of the electrons from one side to the other. The flow of charge ALWAYS goes from the cathode (the positive end) to the anode (the negative end).

Now that we are armed with the basics, here’s how Walt built his galvanic cells. For the anode (or source of negative charge) he uses a galvanized metal, specifically zinc, which they get by collecting coins and spare metallic parts (nuts, bolts, washers, etc). For the cathode (the positive charge where the current will flow out), Walt uses graphite and mercuric oxide that he ground down from the Winnebago’s brake pads. For the electrolyte, he soaks a sponge in potassium hydroxide (KOH). So in this case, referring back to the picture of the galvanic cell, the flow of positive charge will come in the form of K+ ions, and the negative charge from the OH– ions. Remember, we also need a conductor to carry the current of electrons—the charge—from one half-cell to the other. Walt uses copper wire, which he then connects to the jumper cables by collecting all six cells and pooling the electric current to restart the van’s battery.
Realistically speaking would this actually restart the camper? Sadly, probably not. We’ve talked about what a galvanic cell is, now let’s talk about how it works. The charge, or electric current, is generated by two separate chemical reactions (or half-reactions) that occur on either electrode of the cell. At the anode, an oxidization reaction strips electrons from the electrode (usually a metal of some sort), resulting in overall negative charge. At the cathode, free electrons that have traveled through the conducting circuit are used to reduce the electrode species to generate a positive charge. Taken together, these two values add up to the total cell potential, defined as the ability to force electrons through a circuit, and measured in voltage.
The zinc-carbon cell that Walt has built is a variation of a classic Bunsen cell, and we can estimate the cell voltage at around 2 total Volts. If we break down the two half-reactions, oxidation and reduction, we can add up the total potential using a fancy-schmancy mathematical equation called the Nernst equation. The potential of the mercuric oxide reduction, used in commercially available mercuric oxide batteries, is 1.35 Volts, and the potential of zinc oxidization is -0.76 Volts. To determine the full potential of a cell, we subtract the anode from the cathode, and this reaction adds up to 2.1 Volts. Walt mentions that he only has material enough for six cells. So if we add this up, we get enough voltage, give or take, to build a typical 12 Volt battery. Perfect in theory. But, when we talk about real-life circuits, we have to factor in electrical resistance, the opposition of electric current. Resistance occurs in all sorts of conductors, including metals (due to electron scattering) and ionic liquids (depending on concentration and insularity of the solution medium). If we’re going to get super-technical, we could invoke a physics property called Ohm’s Law which states that the ultimate current (which we measure in amperes) potential is inversely proportional to its resistance. The higher the resistance, the lower the ultimate current. So while Walt was able to build a battery with the proper voltage, the internal resistance would likely not generate enough current to provide the immense power necessary to jump-start a car that big. Most cars use a standard 12 Volt battery, but for cold-cracking need about 400-600 Amps, higher if you are going to start something like the Winnebago. The battery that Walt built would probably generate 20-30 Amps of current, based on current for a typical basic Galvanic cell, and that’s being generous. But how many shows on the air are even attempting this kind of clever science? So Breaking Bad gets an A for effort and that’s just all there is to it!
Incidentally, you can easily make your own galvanic cell at home. Check out this neat video of six lemons powering a low-wattage LED bulb:
Lemon Battery – Watch more funny videos here
3) What’s your name?
Of course, every drug kingpin worth his weight in meth has to have a nom de guerre. Mr. White’s sobriquet choice? Heisenberg. I must admit my inner chemistry geek did major cartwheels when I heard this. It’s such an appropriate name for him on so many levels. Heisenberg, of course, is a tongue-in-cheek reference to Werner Heisenberg, one of the great physicists of our time. He is the father of the “Heisenberg Uncertainty Principle” in quantum mechanics, which states that measured values for a particle’s position and momentum cannot be ascertained simultaneously with equal precision. It turns out that particles actually act more like waves, whose three-dimensional spatial function can be separated into three axes, or directions, x, y and z, horizontal, vertical and diagonal, respectively. So, if for a certain particle we can specify momentum with absolute certainty, then its position can be found anywhere along those axes with an equal probability. For this contribution, Werner won a Nobel Prize in physics in 1932.
What a clever and ironic name for Walt, whose own life and identity faces so much uncertainty as the show unfolds: in his cancer diagnosis, his long-term prognosis, his deteriorating relationship with his family, his precarious one with Jesse, and the theoretical uncertainty of his motivations for manufacturing meth, which certainly evolve over the two first seasons.
It’s important to note Heisenberg is also a very controversial historical figure. He played a prominent role in the German nuclear energy project, the race to develop an atomic bomb prior to World War II, although never formally affiliated with the Nazi movement. He later joined a prominent group of scientists who opposed the use of tactical nuclear weapons as warfare. The Tony-award winning play “Copenhagen” centered around the possibilities of a mysterious meeting in 1941 between Heisenberg and fellow physicist Neils Bohr in German-occupied Copenhagen around which there remains to this day… well… uncertainty.
A big ScriptPhD props to the writing team on Breaking Bad for this little gem!
2) It’s enough to just melt you!
Not since the CSI Season 2 episode “Bully For You”, in which a victim’s body found in a duffel bag decomposed so badly it had to be poured out, has a liquefied body played such an important role on a television episode. You’ve just killed the drug dealer who was supposed to peddle your batch of crystal meth, but you have to make the body disappear quietly without your DEA brother-in-law finding out. I mean seriously, folks, we’ve all been there, right? “The best thing to do,” Walt concludes, “is dissolving [the body] in strong acid.” We’ll talk more about this in a second, but first, check out the minisode for the episode “The Cat’s in the Bag…”. Pay particular attention to the disastrous results of this suggestion in the last 1:30, especially if you have a strong stomach.
In the episode, Walt asks Jesse to pick up a plastic container in which to dissolve the body. Even Jesse, dubious of this, says to him, “Any decent acid is gonna eat right through this.” “Not hydrofluoric [acid],” Walt concludes. What? An acid that can eat through flesh and bones but will leave a flimsy plastic bucket intact? This sounds like crazy talk! Well, actually, he’s right. An acid, chemically speaking, is nothing more than a compound that is able to give up a proton to a willing base in solution. By proton we mean a positively-charged hydrogen (H+) with water acting as the accepting base in most cases. The stronger the acid, the more readily it gives up this proton, and the higher the concentration of H3O+ protonated water ions floating in solution, called the dissociated state. Why wouldn’t it react with plastic? Walt tells Jesse to look at the bottom of the bins for a symbol called “LDPE”. He’s talking about low-density polyethylene, the plastic polymer that makes up everything from plastic bags, various containers, dispensing bottles, wash bottles, tubing, to molded laboratory equipment. The repeating units of CH2, some linked by side branches, make for a strong, compact bond and very low reactivity. That is, this chemical will neither give up its hydrogens when exposed to a solution, nor will it accept any. In this case, the acid will primarily disintegrate the body, which as we know is about 80% water, thus creating a solution, but the nonreactive hydrocarbon of the bin would remain chemically inert.
Hydrofluoric acid, contrary to Walt’s statement, is NOT really that strong of an acid, compared to other options, but has some interesting properties useful for Walt and Jesse’s predicament. Back up for a moment. There’s two ultimate determinants of acid strength—electronegativity (the charge on the molecule that the hydrogen is attached to) and the size of the atom that the hydrogen is attached to, which has to do with the strength of the bond. As you move across the periodic table, atom charge strongly increases, which is why fluorine is a much better acid candidate than nitrogen or an unreactive atom like carbon. BUT, as you move down the periodic table, elements get bigger and bigger, and as their size increases the strength of the bond with hydrogen weakens for various reasons. (We’ll skip discussion of covalent bond orbitals for next time!) So acidically speaking, HF < HCl (stomach acid) < HBr < HI. Personally, I really would have chosen a very concentrated hydrochloric acid or sulfuric acid solution like John George “The Acid Murderer” Haigh, or better yet, liquid lye (the base NaOH), which would be able to react with the organic components of tissue and fat content and wouldn’t have dissolved Jesse’s tub, but where’s the drama in that? Realistically speaking, HF would not be able to liquefy the body to the substantial extent shown in the episode. But because it is a weak acid and exists primarily in its undissociated state, it is able to penetrate deeply into the skin before deprtotonating, thus making it an excellent and efficient corrosive for human flesh (and very dangerous!). That’s right, it eats your body from the inside out. More importantly, it reacts strongly with calcium and magnesium, so it would be able to efficiently dissolve Emilio’s bones for disposal. [Incidentally, when people are treated at the hospital for HF poisoning, the first line of treatment is calcium gluconate to “compete” with the calcium in your bones as a neutralization reaction.] Unfortunately, it also reacts with silicon dioxide, the major component of glass and ceramics. Hence the look of absolute and profound horror on Walt’s face as Liquid Emilio, the bathtub, and everything in-between come crashing down.
Take home lesson? If you’re desperate to get rid of a dead body by chemical disincorporation, for God’s sakes, read up on your chemistry first!
Annnnnnd ::drumroll:: the best Walter White chemistry moment to date?
1) A little tweak of chemistry!


Without question, the scientific highlight of the show to me, thus far, is the integration of clever reaction chemistry to advance a major plot on the show. Early in the episode “Crazy Handful of Nothin’”, Mr. White is teaching his class about the power of chemical reactivity. Sometimes, it’s gradual and imperceptible, to which he gives the example of metal oxidation. But other times, the reaction is violent, quick, and produces tons of energy. On the chalkboard, he writes an example of an explosive compound, mercury fulminate, Hg(ONC)2. Relatively easy to make synthetically, fulminated mercury is a powerful explosive and was long used as a detonation primer for dynamite.
This chemistry lesson proves to be a dynamite primer of its own later in the episode. The first good large batch of meth—1 pound!—that he and Jesse were able to synthesize was stolen without compensation by Albuquerque’s toughest drug wholesaler. What’s a wronged high school chemistry teacher to do? Walt shows up at Tuco’s casa with a large crystal of what looks like more meth and a demand for his money. But Walt surprises him by announcing that it isn’t in fact meth, and throws the crystal to the ground. The result? KABOOM! See the before and after pictures. What is that stuff, a stunned Tuco asks. “A little tweak of chemistry.” Most notable about this scene is that it caps the season-long transformation of Walt’s character. As he walks over to his car, money in hand, reputation restored, he hasn’t just earned the respect of a thug. This is the moment in the show that delineated a before and an after, where the line was drawn in the sand, and we never again saw the innocent sick chemistry teacher desperate to save his family. A drug dealer was born.

The only slight criticism that I have is that in reality, mercury fulminate wouldn’t actually look like the large meth-like crystals portrayed on the show. The chemical synthesis process usually leads to very fine white powder precipitate crystals like the one in the picture on the right. But we can just assume that Walt used his past career as a “Crystallographer Extraordinaire” to produce the largest mercury fulminate crystals to date ;-). Secondly, this stuff is unstable and extremely reactive. As in don’t touch it, don’t expose it to light, don’t mess with it unstable. It can be detonated by sparks, shock, friction, or even a wayward glance. Realistically speaking, Tuco handling the stuff with his knife and dropping it on the table would be enough to ignite it. We’ll also have to assume Walt was very careful in handling an entire plastic bag of it. Nevertheless, this whole scene is so creative, well-written and downright badass, that here at ScriptPhD.com, we can’t allow minor quibbles to detract from the moment.
The season finale of Breaking Bad aired May 31, 2009 on AMC, but for those of you not yet watching this spectacular series, there is plenty of time to catch up. The Season 1 DVD is available in stores and the Season 2 DVD release date will be updated on our site as soon as we know it! And we’re going to do our darndest to welcome Vince Gilligan, the show’s brilliant creator and executive producer, to our in-house ScriptPhD lab to chat all things Breaking Bad! Stay tuned!
For a look ahead at Season 3, check out this sneak peek preview:
All video clips and pictures are © 2007-2009 AMC Television and Sony Pictures Television. All rights reserved.
~*ScriptPhD*~
]]>