
One of my favorite movies as a kid, and now, as a professional scientist, is Andromeda Strain. The heroes are mostly older, professorial types who work feverishly to understand an alien organism and save the planet. After being asked to review ReAction! Chemistry in the Movies for ScriptPhD.com, I was so curious to consume (with relish) the book’s guesswork about the chemistry found in Andromeda Strain. After returning to the beginning of the book and giving it a read, I was thrilled to find that ReAction! is a detailed, thoughtful exploration of the representation of chemistry in film. The book addresses, first and foremost, the fact that chemistry can play a lead role in film. The authors also discuss the dichotomy between the “dark” and “bright” sides of chemistry (and science) as illustrated by films in which chemistry or chemists play a central role. Also included are several playful explorations of the real science behind some famous examples of fictional chemistry in film. After the break is a full review of the book along with an in-depth interview with authors Mark Griep and Majorie Mikasen on the process of working together as chemist and artist, portrayal of chemists in film and how film can change public perception in science.
Often referred to as the “central science,” chemistry (and chemists) have a rich history of adapting to focus on the problems of their day. Therefore, chemists often make breakthroughs that are remembered as medical, technological or biological. Penned by chemistry professor Dr. Mark Griep and his painter wife Marjorie Mikasen, ReAction! points out that many examples of science in film are, in fact, misclassified as chemistry. Chemistry, the authors suggest, seems to occupy an interesting and unexplored niche in film; ReAction is the culmination of their years-long quest to understand how chemistry is portrayed in film. Intriguingly, the book is also an exploration of the history of chemistry as a discipline as reflected in the film, charting the course from the creation of the periodic table to chemical synthesis to modern drug discovery.
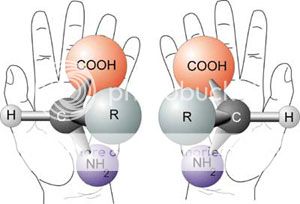
An important theme of the book involves the stark difference between the use of chemistry for “dark” or “bright” purposes. The authors’ view is that this duality underpins how society views science, as both great and terrible. One of the most well explored “dark” films of the book is Dr. Jekyll and Mr. Hyde. While the basic story is nearly universally known, the authors explore its film representations carefully, exposing both chemical and cinematic features of interest. The mirror-like duality between Jekyll and Hyde is used as a vehicle to confront the concept of chirality, which is the notion that molecules can have non-superimposable, mirror image isomers. As the authors undertake an exploration of the fictional chemistry of Jekyll’s formula, a stunning amount of chemistry is discussed. Jekyll’s formula contained “a white crystalline salt, blood-red liquor and a mysterious impurity.” The blood-red liquor, consisting of red phosphorous dissolved in carbon disulfide, introduces both nanoscience and stoichiometry. A discussion of the white crystalline salt centers on the observation that Dr. Jekyll and Mr. Hyde’s author, Robert Louis Stevenson, likely suffered from a bleeding disorder. At the time, the best treatment and likely identity of the salt was a water extract of the ergot plant. This, the authors argue, means the unknown impurity was probably ergotamine. The historical developments surrounding ergot leads to a discussion of the beginnings of the pharmaceutical industry and introduces the concept of plant-derived drugs. Interwoven in the science is, of course, a discussion of the dark themes of self-experimentation and violence.
In this clip from the original classic 1912 silent movie, Boris Karloff transforms from Dr. Jekyll into Mr. Hyde (and back):
On the “brighter” side of things is Dr. Ehrlich’s Magic Bullet, a film that tells the story of the extraordinary scientist Paul Ehrlich. Ehrlich won the Nobel Prize in Physiology or Medicine in 1908 for important contributions toward understanding the immune system, and also developed the first synthetic chemical treatment for disease. The authors describe how Ehrlich developed his “side chain theory,” which suggested that drugs and receptors interact in a specific way. This theory led Ehrlich to realize he could start with a “lead compound” that was weakly effective, or even toxic, and develop it into a powerful drug. Ehrlich started with the syphilis lead compound atoxyl and found a derivative, salvarsan, which was the first effective syphilis treatment. The magnitude of this discovery cannot be understated—Ehrlich’s methods ushered in the era of modern drug discovery and because Ehrlich was working at a time when the thought of injecting chemicals into humans to treat disease was unusual.
ReAction! gives a detailed analysis of the science and history of science related to many of the chemistry-related issues raised by the films it discusses. In fact, the science concepts introduced range from the very simple (stoichiometry) to the very complex (statistics); some of these concepts might be a bit difficult for a lay audience without a scientific background to grasp. However, the overall impression is that the authors have succeeded in using film as a vehicle to impart an incredible amount of science.
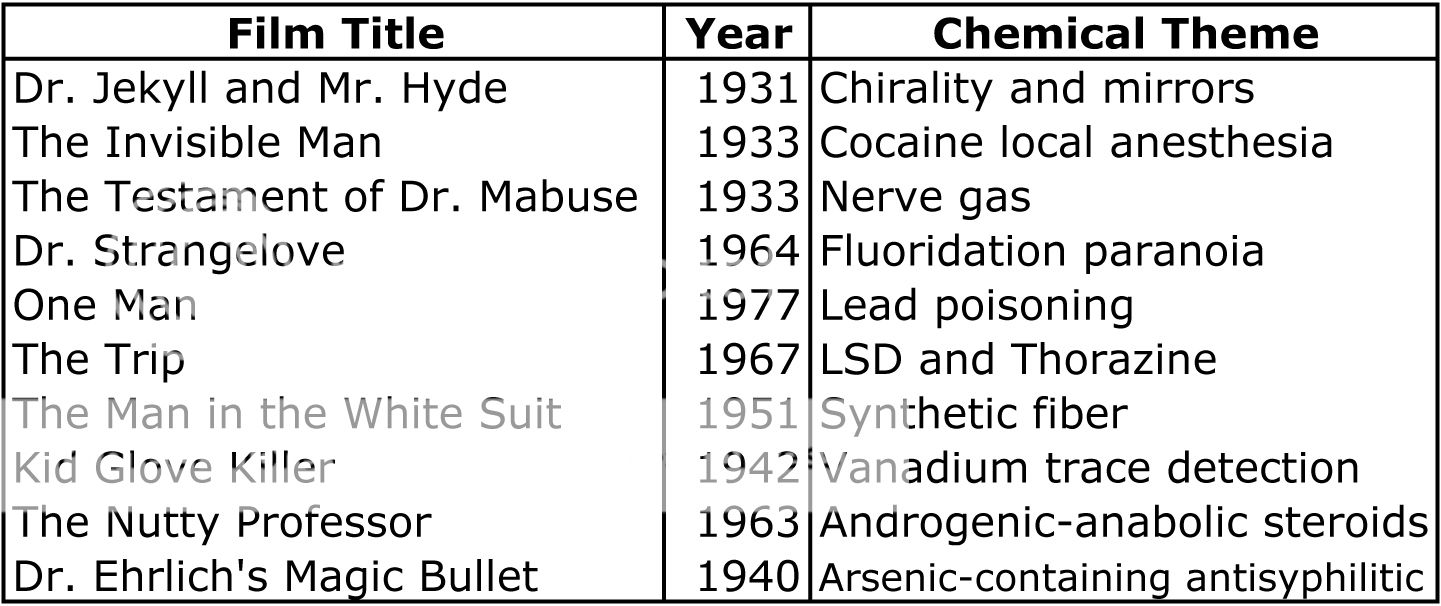
When I first picked up ReAction! and tried to think of examples of chemistry in film, I didn’t have too much success. Like the authors, I initially called to mind the myriad examples of technology, biology and medicine that are present in film. But after reading ReAction!, I’m sold on the idea that a rich tradition of chemical themes is present in film. At the end of the book, the authors give a list of 10 archetypal films that have chemical themes (table on the left). I’ve seen a few of these films, but now, I’m eagerly looking forward to watching the rest! And what about the chemistry of the alien microorganism in Andromeda Strain? I’m sad to report that ReAction! reveals that the mass spectral analysis in the film reports an elemental composition that can’t exist. After a bit of tweaking, the authors suggest that perhaps the alien life form could be alkaloid-like, and that the rock-like substance the life form arrived on could be a siloxane. It turns out the science behind that bit of Andromeda Strain wasn’t so sound; I guess you can’t have it all…
I caught up with Dr. Griep and Ms. Mikasen for an in-depth interview about their book, the portrayal of scientists in film, and the social outreach responsibility of scientists to enable better public perception of science.
Doug Fowler: I’m fascinated by collaborations between artists and scientists, which are often intriguing. Can you tell us how you decided to write ReAction!, and write it together?
Marjorie Mikasen: The first thing you have to know is that we are married and that we’ve been enjoying movies together since our first date. We prolong the movie experience by discussing them afterwards. Cut to 2000 when we rented the Elvis movie Clambake to take our new TV out for a spin. It was in Technicolor. Imagine our surprise when, one hour into the movie, Elvis was revealed to be a chemist on the run from his father’s oil company. The Elvis character was developing a superhard, superfast-drying varnish that he called GOOP. In a song, he sings its name as “Glyo-Oxytonic Phosphate”. We thought it was clever and funny that varnish is sometimes called goop and that the screenwriter turned the nickname into an acronym.
The impetus for the book started during the next few months. Mark is a chemistry professor at the University of Nebraska-Lincoln. Since he knows the chemistry naming rules, he wondered whether he could draw the structure for Glyo-Oxytonic Phosphate. He couldn’t. A few months later we watched the movie again in hopes there were other clues. Surprisingly, another character clearly enunciates its name as Glyol-OxyOctanoic Phosphate, a name for a structure Mark could draw after assuming “oxy” is short for “epoxy” and that the compound was designed in analogy to linseed oil, a real varnish. After watching Clambake, it seemed every third movie we rented had some chemistry in it. After collecting about 20 examples and searching for common themes among them, Mark decided to keep a list so he could search movie encyclopedias and online databases. We began purchasing movies with chemical themes. The tipping point for writing the book occurred when we realized the Alfred P. Sloan Foundation had a Public Understanding of Science program for movie-related projects. We were able to persuade the program officer that a book such as the one we wanted to write would be useful for chemistry instructors who wanted to inspire students to want to learn more chemistry. We were delighted that Oxford University Press wanted to publish such a book.
DF: How did your two very different points of view come in to play in considering how to approach the subject?
MM: After we decided to write the book, we had lunch together once a week to discuss it. Mark had already assembled about thirty common chemical themes found in the movies. In half the cases, chemists or chemistry was portrayed as a bad thing and, in the other half, they were portrayed as good. Keep in mind that Elvis used his new varnish to win a boat race, which allowed him to win the affection of his girlfriend and the respect of his father. It was easy for the two of us to select the six or seven strongest themes out of the thirty.
Mark Griep: Marjorie is an artist who is always playing ideas and themes off one another. In her consideration of other types of dualities, she saw that Jekyll and Hyde is the chemical embodiment of benevolence and darkness so it quickly became the book’s overarching theme. With that in place, we developed the structure of five dark themes (Jekyll & Hyde, Invisible Man, chemical weapons, bad chemical companies, and illegal drug use) followed by five bright themes (chemical inventors, chemical detectives, chemical instruction, benevolent chemical researchers, and drug discovery). The actual order of the chapters was deliberately chosen to emphasize the dualities between the two halves of the book. For instance, the difficult-to-detect invisible man is the second chapter on the dark side while chemical detectives are the second chapter on the bright side.
DF: I read that the book was funded by an Alfred P. Sloan grant for the Public Understanding of Science. Is the book part of a lifelong interest in public outreach?
MM and MG: Artists and Scientists are always educating the public about their work. Both of these fields are at the edge of society’s quest for understanding so it is important for their practitioners to discuss their exploration of the frontiers. This book isn’t our first collaboration but it does represent our biggest co-venture. With our book, we wanted to use feature films to show the wider public how chemistry is an integral part of our society and how real chemistry ended up in the movies. Like it or not, movies are mediators of public understanding. They selectively portray things about society and, because of their wide dissemination, they have the power to influence many people. It is important for our society to have a scientifically literate public and we thought this would be a fun way to generate a thirst for more scientific knowledge.
DF: How did you decide to focus on chemistry found in movies as opposed to more mundane but realistic venues?
MM: Mark found that students responded to the chemistry in movies much better than they responded to the chemistry in news reports. When Mark taught General Chemistry for the first time, he gave the students a 600-word writing assignment. They were supposed to write about the chemistry in a recent newspaper or news magazine. Such an exercise promotes deep learning because the student has to process the issues and decide what is important. Mark was surprised that only 60% of the students completed the assignment and disappointed that some of them wrote about topics such as vaccines, supernovas, etc. without mentioning their chemical aspects. Mark decided he needed more control over the student choice of subject material. By that time, he had a list of based-on-a-true-story movies that were chemically inspirational. The next two times Mark taught General Chemistry, he projected two of these movies and had the students write about one of them. It was a great success; 95% of the students completed the assignment and the quality of writing was vastly superior. To make it easier for other instructors to repeat this blockbuster of an idea, we published a summary of it in the Journal of Chemical Education that included a list of the chemical and societal themes for 12 movies based on true chemical stories.
DF: Throughout the book, Dr. Jekyll and Mr. Hyde was the most discussed prototype movie. I wondered how you made the decision to make that movie central to the book. Is it a movie you particularly like or have a personal connection with, or is it just because it’s such an old, publicly recognizable movie involving chemistry?
MM: Like everyone else, we knew the phrase “Jekyll and Hyde” before we ever read Stevenson’s 1886 book or saw one of its many movie adaptations. The first dramatic version we watched was the 1931 version starring Fredric March. His transformation scene is enjoyable because it is theatrical. You can imagine it would be an interesting challenge for an actor to change personalities to match his appearance and March does a great job.
Even though Jekyll is shown mixing chemicals, Mark was intrigued that the transformative formula was not described in enough detail to know what it was supposed to represent. Stevenson’s original story describes a contaminant in a white powder that changes color when it is added to a blood-red solution but it doesn’t name the powder or the contaminant. Seeking other avenues to pursue, Mark contacted Stevenson scholar Richard Dury to find out whether any scholarship had been done on this topic. Dury told him that Stevenson’s wife Fanny had written a letter immediately before Stevenson wrote the novella in which she says he suffered from hallucinations after being treated with an ergot extract. This episode appears to have inspired Stevenson to write the story. Mark then discovered that ergot fungus is a pharmacological toolbox containing compounds to constrict arteries but also compounds to cause hallucinations. It seems that Stevenson’s doctor had treated him with the ergot extract to stop the bleeding in his lungs. The hallucinogenic side effect was caused by a minor component of the extract. This led Mark to conclude what Jekyll’s compound might be.
MG: When we
began preparing to write our book, Marjorie pointed out that Jekyll’s physical transformation was the external manifestation of his personality change. Mark responded that these transformations link it to chemistry because chemistry is about the study of matter and its transformations. Marjorie suggested using Jekyll and Hyde as the overarching theme for the book because the character of Jekyll/Hyde transforms between a dark side and a bright side, giving us a way to describe the positive and negative depictions of chemistry in the movies as a whole. As we further honed the chapter structure of the book, we knew there were going to be many echoes of the Jekyll and Hyde. In fact, anyone who self-experiments in the movies is following in the footsteps of Dr. Jekyll.
DF: My favorite movie about scientists is Andromeda Strain, because it portrays a relatively realistic view of who scientists are and what they can accomplish. However, in most movies, scientists and their abilities are rendered in a much more dramatic, unrealistic style. How do you think this affects the public’s view of scientists?
MM and MG: It is important for the pace of a feature film to use stereotypes. It helps the audience get into the story quickly so they can watch the interaction between the characters rather than the actors.
How do you recognize a chemist in the movies? When the chemist character is an inventor, he wears a white lab coats as he handles beakers and flasks while solutions bubble in glassware behind him. He often accidentally synthesizes a compound that solves a personal problem. These sorts of films are still being made and show an archaic view of the modern laboratory where nearly everything is on a small scale and instruments are used to analyze the material.
When the chemist character is a criminologist, he or she wears a white lab coat while using microscopes and other instruments to examine the minutiae of a crime scene. The solution to the crime, however, usually relies upon intuition. Chemically determined facts are used to eliminate possibilities. These chemists understand chemical analysis and criminal behavior.
When the chemist character is a researcher, he or she searches for a solution to a problem affecting other people. Most of the research chemists are based on true stories and aren’t as popular as the above two types of movies. They tend to move at a slower pace because the nature of the science and tools has to be explained in an engaging but understandable way. They are usually biographical pictures.
These three chemist stereotypes give us the shorthand view of what fictional chemists look like and their motivations for doing research. All three types are obsessive about their work, which is not too far from reality. In general, we think these stereotypes are benign.
DF: Does it help or hurt the cause of publicizing science?
MM and MG: Let’s just say we will always need good educators to clear up these sorts of misunderstandings. It would be great if we could teach people to be optimistically skeptical when they encounter new claims. Movies and television are problematic in that viewers are able to see that something works with their own eyes. Even if they know it is “only a movie” or “only TV”, the image has entered their memory cells. If it is reinforced in any way, they will remember the image long after they’ve forgotten its source. As long as academicians keep putting out good information in reliable locations, the public will have somewhere to look when they are seeking answers.
Another answer to this question is that it helps and hurts. Consider the “CSI Effect” that trials have been experiencing in recent years. It seems that juries are demanding more sample analysis than the investigators carried out. For instance, juries might want to know why DNA analysis wasn’t performed on all samples at the crime scene. The people serving on juries don’t realize how expensive or time-consuming it is to run each analysis. The positive spin is that juries want to have all the dots connected by solid scientific methods. It is great they want to rely on physical evidence rather than on unreliable eyewitnesses.
DF: Your book touches on the fact that chemistry has also changed a lot over the course of recent history. Can you expand on how these changes are reflected in the portrayal of science and scientists in the movies?
MM and MG: The portrayal of chemists and chemistry in the movies has become much more realistic in recent years. Since comedies often rely much more heavily on stereotypes, they mirror their time really well. In the 1930s comedies such as The Chemist (1936) starring Buster Keaton and Violent is the Word for Curly (1938) starring the Three Stooges, the chemist characters are professors who wear a black mortar boards and capes rather than white lab coats. Of course, these slapstick artists were making fun of the arcane knowledge produced by higher education. Flash forward to the 1990s when we had The Nutty Professor (1996) starring Eddie Murphy and Flubber (1997) starring Robin Williams. There is still too much glassware and these inventors are still University professors but they use correct scientific terms, have appropriately outfitted laboratories, and colleagues who interact with them realistically. These 1990s comedies were made for the youth market, none of whom would know whether these things were correct or not. These comedies use the reality of factual chemistry and its accoutrements to launch their satirical fantasies.
DF: Chemistry is often defined as “the central science,” and chemistry seems to influence many different fields of science. As such, it’s often hard to know just what qualifies as chemistry. How did you draw this distinction when classifying movies?
MM and MG: The basic rules of classification are simple. It is the exceptions that are complex. The two simple rules for inclusion are: 1) a character is identified as a chemist, or much more rarely, a biochemist, geochemist, etc.; and 2) one of the characters mentions an element, isotope, compound, or simple mixture. The characters are further categorized according to the type of chemistry they do. The chemicals are usually categorized by name. Overly common chemicals, such as gold and water, are not tracked even though one is an element and other is one of the most important compounds on Earth. On the other hand, when gold plays a role in a movie that has other chemical features, the gold is tracked. For example, munitions maker Tony Stark (Robert Downey Jr.) isn’t an iron man for very long in Iron Man (2008). For most of the movie, he’s actually gold-titanium alloy man whose suit is powered by a plutonium-powered arc.
DF: Aspects of science and the scientific establishment have come under increasing attack by various organizations and individuals. Public distrust of science and scientists seems to be increasing, whereas scientific literacy has decreased. What role do you see movies and other media playing in these trends?
MG: Before I answer the question, I’d like to disentangle a few issues. The National Science Foundation published an interesting study in 2002 titled “Science and Technology: Public Attitudes and Public Understanding”. There have been many changes since 2002 but the public probably still believes overwhelmingly that scientists wear glasses and white lab coats while working with beakers and flasks for endless hours alone in the lab. On the other hand, the public also says that “scientist” is the second highest prestige occupation (56% enthusiasm), just behind “doctor” (61% enthusiasm). The public respects science although they don’t necessarily want to be a scientist. The NSF article also noted the public thought economic and educational issues were more important than global warming. It is not really a contest to determine which of these three topics is of greatest interest to the public but it does say most people are most interested in issues that touch closest to home. Knowing that the average global temperature is slowly rising is only part of the equation. People want to know what they can do to adapt now in a way that doesn’t harm them economically. I don’t think that message has been communicated at all.
I agree that scientific literacy has decreased but there are many factors at play. One is that people rely less on their memories today and more on the internet for factual knowledge. For instance, you can learn an awful lot by reading Wikipedia, but you should always verify the cited sources. Another factor is the sometimes incredible expectation raised by the science in TV shows, movies, and news articles. As authors of a book about chemistry in movies, we see this as a great opportunity for a teaching moment. It is one of the main reasons we wrote our book. Yet another significant factor is that science is taught as a collection of facts rather than as a method of discovery. There are many ways to solve the latter problem. One is to educate students how to develop and test their own hypotheses. At first, students would reproduce well-known phenomena but they would develop an appreciation for proper methodology and the power of science to test a hypothesis. These hands-on experiences could be coupled with student-centered instruction where groups of students work together to answer a series of factual questions. In this way, the students take possession of the knowledge. Finally, introductory science material should be presented as a series of tested hypotheses rather than as building up from basic established principles. Introductory courses should explore local and global issues so that students can see that science is being used to address the biggest questions of our time. All of this would help students experience the wonder of science, where your understanding of something changes after you learn something new.
It may seem like a contradiction to what I have just written but feature films can be used to help teach chemistry. The movies in our book were selected because they present chemistry in a social context, even when the movie is fictional. The dozen movies that are based on true stories about chemists can be shown to students in their entirety. The chemist character’s social hurdles are emphasized along with the scientific ones. The assignment could be to answer a series of factual questions or to write a report with citations that emphasizes the chemical aspects presented in the film. There are often factual inaccuracies or time compressions that gloss over some relevant details. Besides these biographical movies, there are several dozen movies with a 2- to 5-minute sequence in which the chemistry that drives the narrative is explained. I call these the scientific-explanation scenes. They tend to be funny or exhilarating. In our book, the cue time for each of these scenes is indicated in the narrative summary, along with descriptions of the real chemistry upon which it is based.
Douglas Fowler, PhD is a National Institutes of Health Postdoctoral Fellow at the University of Washington working to implement new technologies to investigate protein sequence/function relationships. Although more of a biologist these days, he got a BA in chemistry and philosophy at Northwestern University and a PhD in chemistry at The Scripps Research Institute.
*****************
ScriptPhD.com covers science and technology in entertainment, media and pop culture. Follow us on Twitter and our Facebook fan page. Subscribe to free email notifications of new posts on our home page.