
…a nanoscientist’s quest to mimic Nature’s molecular blueprints
Have you ever found yourself entranced by the exquisite beauty and complexity of living things? Like the intricacies of a budding flower, or the mesmerizing patterns on a butterfly’s wing? Have you ever wondered: “what are living things made of?” Are these materials just as beautiful if we were to zoom way way in and look at the actual molecular building blocks that make up life? Take a look at the interactive link The Scale of Things to see just how small the building blocks of life really are! Well the answer is “OMG – totally!” All living things share a ubiquitous set of molecular building materials we call proteins, and they are absolutely stunning! They are not only smashingly beautiful to look at, they are capable of performing a mind-numbing myriad of very intricate and complex functions that are essential to life. In a very special guest post, leading nanoscience Professor Ron Zuckermann of the renowned Lawrence Berkeley National Laboratory recounts his life’s mission as a chemist to try and build artificial microscale sheets made up of nature’s very own building blocks—proteins. Everything you wanted to know about what nanotechnology is, exactly, why engineering proteins is the science of the future, and what we plan to use these discoveries for, under the “continue reading” cut.
I am a Materials Scientist working in a nanoscience research institute called The Molecular Foundry. A fundamental problem in nanoscience is how to make man-made materials with a similar level of precision and complexity at the molecular level found in nature. I am interested in applying lessons from the world of protein structure to practical man-made materials. If we are successful, we should be able to make materials that are cheap and rugged like a piece of plastic, yet be able to perform highly sophisticated functions, like recognizing a molecular partner with high specificity, or even catalyzing chemical transformations. Such materials could be used to make sensors for the detection of harmful chemicals, or as robust medical diagnostics that could survive harsh conditions, say in an underdeveloped nation. In a nutshell, we aim to make artificial proteins. This is an incredibly difficult problem, and one I have been working on for more than 20 years now. Sound a bit ambitious, or maybe a bit crazy? As I will describe in this article, it may actually be quite possible if we break the problem down into bite size chunks. The challenge comes down to two fundamental things: design and synthesis.

Protein architecture
When I look at the molecular structure of a protein molecule, I see an architectural blueprint that has survived untold generations of evolution and optimization. How then do we break open the hidden rules in these structures and use them to guide us in the design of man-made materials? Over the past several decades, scientists have used the biophysics techniques of X-ray crystallography and nuclear magnetic resonance spectroscopy (NMR) to determine the exact three-dimensional (3D) structure of thousands upon thousands of proteins. What’s cool is that these are all available for anyone to look at (for free!) and study in the Protein Data Bank.
The most fundamental thing to notice is that nearly all protein structures share the following characteristics: (1) they are made of a linear polymer chain that is folded into a precise 3D structure and (2) they are comprised of only 20 simple molecular building blocks called amino acids, arranged in an exact sequence along the polymer chain. When we think of a ‘polymer’ we think of a long chain of repeating chemical building blocks (called monomers) found in materials like nylon or polyethylene. Such man-made materials are incredibly useful and ubiquitous now in our environment (plastic bags or saran wrap, for example). But nature beat us to the punch a long, long time ago. Biopolymers, like proteins and nucleic acids are fundamentally way more sophisticated than man-made polymers. Even though they share the same basic architecture – a linear chain of chemical building blocks – biopolymers contain information encoded in their monomer sequence. This is not unlike the way we store information in a computer. But instead of a long string of 1’s and 0’s, nature uses long polymer chains of either 4 nucleotides (the building block units of RNA and DNA), or 20 amino acids (the building block units of proteins). These 20 chemically distinct amino acid building blocks are arranged in a particular order along the chain that we refer to as the protein’s “sequence.” This sequence is powerful because in many cases it provides all the information or “molecular instructions” necessary for the polymer chain to fold up into a precisely defined 3-dimensional structure. Once folded, the protein is poised and ready for action. The fields of Structural Biology and Protein Folding have revealed the exact way that proteins fold to form local “secondary” structures, called alpha helices and beta sheets, and how these assemble together to create the fully folded protein structure. Think all this sounds a bit too complicate? Try visiting FoldIt, a really fun video game where you can actually learn all about protein folding!
Protein Mimicry
If we ever hope to create man-made protein-like materials, it is safe to assume that we will need a polymer system that shares some of the basic protein-like characteristics: for example, they will need to have a sizable set of chemically diverse monomer building blocks that can be arranged in a particular order along a linear polymer chain of at least 50 monomers long. This is a quite a tall order simply from a chemical synthesis perspective. Moreover, once we are over that hurdle, design tools will be needed to help us figure out which sequences to make.
In the early 1990s, I invented a way to synthesize a new family of non-natural polymers we called “peptoids.” I had just graduated from UC Berkeley with a PhD in organic chemistry and joined a start-up biotechnology company to develop new technologies to accelerate drug discovery. We developed peptoids to be potential therapeutic drugs. The cool thing about peptoids is that the building blocks are very very close in structure to Nature’s amino acids, but different enough to be much more rugged. They can be made from very cheap and simple chemical building blocks, and they can be made in any predetermined sequence you want. We soon developed robotic synthesizers to automatically synthesize these materials for us (see below).

Before long we discovered that short peptoid chains (just 3 monomers long) could have potent biological activities and showed promise as drug candidates. But what really floored me was that the peptoid synthesis chemistry we developed worked so efficiently that we could link over 50 monomers together, one after the other. This means each building block was being attached to a growing chain with an incredible accuracy of over 99.5%! This was exactly the tool we needed to begin the quest for creating artificial proteins. We had discovered the most efficient and practical way to make non-natural polymers of a specific sequence. This was completely awesome!
There was only one problem – the company I worked for had no interest in such a bold quest into basic science. How could such a pursuit lead to a moneymaking product in a few months? In 2006 I moved to Lawrence Berkeley National Laboratory where I set out in earnest to search for artificial proteins. Fortunately, tackling difficult problems in basic science is much more the norm here. And more good news – my previous employer was kind enough to donate my robots to me. Armed with this technology to synthesize peptoid polymers, we turned to the next daunting task: which sequence of monomers should we make? It turns out that there are an absolutely astronomical number of possible peptoid sequences that can be made. Consider that there are several hundred building blocks to choose from at each of the 50 positions of a polymer chain. This means there are over 100 to the 50th power potential sequences to choose from. This is more than the number of atoms in the universe! What was a chemist to do?
Like Oil and Water
To help us focus our sequence design, we once again turned to nature. A long-time collaborator and friend of mine, Professor Ken Dill of UC San Francisco has studied protein structure in detail for decades, and has distilled some fundamental rules that are universal to all protein structures. He notes that protein structures are like globes with a water-loving surface and a water-hating (or oil-like) interior. The bottom line is you can basically lump each amino acid in the protein’s sequence into one of two categories: oil-like or water-like. This simple classification can tell you whether an amino acid is located on the inside or the outside of the protein.
The amazing thing about this insight is that it’s like looking at a protein wearing X-ray glasses! Instead of seeing 20 different “colors” of amino acids, we see only two: black and white. We are back to a simple binary code—like 1’s and 0’s. This makes it much easier to see the secret patterns hidden within the sequence. In fact, many researches have convincingly demonstrated that these binary patterns are simple, plentiful and predictable.
So with our handy X-ray glasses on we returned our gaze to the peptoid structure problem. We realized that all our sequence recipe needed was a touch of Dill! This meant we could greatly simplify our search for the right peptoid sequence. We needed to only consider two, diametrically opposed building blocks: water loving and water hating.
Nanosheets
Inspired by these insights, we set out to find the right sequence patterns that would result in a precisely ordered structure in a non-natural peptoid polymer. We began to systematically unlock the sequence code by using our robots to synthesize all the possible repeating patterns of these two disparate building blocks. We reasoned that if we were to make something precisely ordered, it would “crystallize” into something that we could see. Fortunately we have really powerful electron microscopes in my building. So we started cranking through all the possible sequence patterns, dissolving up each new sequence in some water and taking it down to the basement to look at them really close up.
Now it is a little nuts to think that we could make something precisely structured from a peptoid polymer, since it is known that each strand is about as stiff as a piece of overcooked spaghetti. And as one might expect, most of our sequences looked really messy and gooey. But very soon we saw something quite striking. In one particular sample, we saw large, flat paper-like objects with sharp, straight edges. And they were floating around everywhere in the solution. An unexpected sight to be sure!
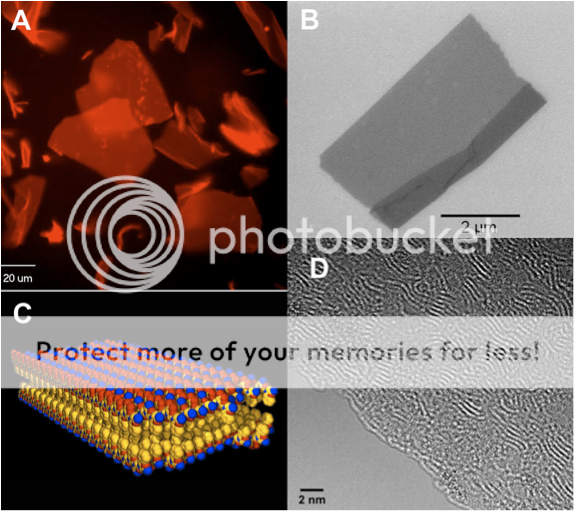
Fast-forward another year of making careful measurements and reproducing the results over and over. We determined that these sheets were only two molecules thick, and yet millions and millions of molecules wide. We had discovered the largest and thinnest organic crystals ever! We were able to use one of the most powerful electron microscopes in the world in the National Center of Electron Microscopy, to look directly at the individual polymer chains that make up the crystal. We could see these chains wiggle around and slide against one another as if they were alive. No one had ever seen such detail before – a truly awe-inspiring sight!
We were able to use many kinds of advanced analytical tools to tell us that these nanosheets were indeed very special. They have the same kind of ordered structure that a protein has: they have a defined inside and outside, and we know almost exactly where each atom is located in the structure. Essentially, we discovered the sequence code that programs the polymer chain to form a 2D sheet. No doubt there are more complex codes waiting to be discovered that will form even more sophisticated structures. This discovery was recently reported in the journal Nature Materials, and was picked up by the more mainstream publications WIRED.com and Chemical & Engineering News. These sheets are likely to be important for all kinds of potential applications. Their discovery is kind of like the invention of ‘molecular plywood’: a new kind of nanoscale building material from which we can engineer even more complex molecular architectures. It’s amazing what kind of beauty you can create from simple building blocks!
Basic research like this can move seemingly very slowly, which makes the occasional breakthrough like this all the more meaningful and exciting. It reaffirms for me that it is so important to listen to and cultivate your inner curiosity, surround yourself with like-minded people, and aim high. With enough patience and persistence, wonderful things await discovery!
Take a look at a brief video of Dr. Zuckermann explaining his lab’s nanosheet discovery:
Ron Zuckermann is the Facility Director of the Biological Nanostructures Facility at Lawrence Berkeley Laboratory. Dr. Zuckerman also provides numerous consulting services at the intersection of chemistry, biology and engineering.
*****************
ScriptPhD.com covers science and technology in entertainment, media and advertising. Hire our consulting company for creative content development.
Subscribe to free email notifications of new posts on our home page.
]]>
ScriptPhD.com is extraordinarily proud to present our first ever Science Week! Collaborating with the talented writers over at CC2K: The Nexus of Pop Culture and Fandom, we have worked hard to bring you a week’s worth of interviews, reviews, discussion, sci-fi and even science policy. We kick things of in style with a conversation with Professor Malcolm MacIver, a robotics engineer and science consultant on the SyFy Channel hit Caprica. While we have had a number of posts covering Caprica, including a recent interview with executive producer Jane Espenson, to date, no site has interviewed the man that gives her writing team the information they need to bring artificial Cylon intelligence to life. For our exclusive interview, and Dr. MacIver’s thoughts on Cylons, smart
robotics, and the challenges of future engineering, please click “continue reading.”
Questions for Professor Malcolm MacIver

ScriptPhD.com: Your first Hollywood science experience involved consulting for a sequel of the 1980s cult classic Tron. What was it like to dive in from the Northwestern School of Engineering onto a
set and work with screenwriters? What were some of your first impressions?
Malcolm MacIver: It was fascinating to learn a bit about how these huge expensive projects are structured. One specific thing I wanted to know more about was the role of writers in movies versus in TV. I had been told by friends in the industry that writers are typically less prominent players in movies than in top TV shows, were they can have considerable power. Consistent with this, we (the scientists who met with the Tron folks) were not introduced to the writers, who I believe were in the room taking notes, while we were introduced to all the other major players (director, producer, etc). I was also very curious to see how the group of scientists that I was a part of would interact with the movie makers. The culture gap is obviously huge, big enough for massive misunderstandings to blossom during superficially neutral discussions. During our meeting, our approach to the folks involved with the movie varied
from inspired to less admirable attitudes. The less admirable attitudes seemed to arise from the mismatch between the importance scientists can place on their own endeavors, relative to their endeavor’s importance to story telling.
An anecdote I like along those lines is about how the astrophysicist Neil deGrasse Tyson complained to James Cameron that when Kate Winslet looked up from the deck of the Titanic, the stars in the sky were in the wrong position. I liked Cameron’s response, which was “Last I checked the film’s made a billion dollars.” People love the story, not the positions of the stars above the Titanic. We all tend to overemphasize the importance of the thing we are closest to, and it’s a problem that scientists need to be especially attuned to in these contexts.
SPhD: You are now a technical script consultant for Caprica, the television prequel to Battlestar Galactica, providing insight into things like artificial intelligence, robotics and neuroscience. To date, what has been one of your biggest contributions to a final written episode that otherwise wouldn’t have made it in to the storyline?
MM: One of the themes of my research is understanding the ways in which intelligence is not just all about what’s above your shoulders. Nervous systems evolved with the bodies they control—the interaction is extremely sophisticated, and stubbornly resists our attempts to understand it through basic science research or emulation in robotics. Representative of this fact is that we now have a computer that can beat the world chess champion—a paragon of an “above the shoulder” activity—while we are far from being able to robotically emulate the agility of a cockroach.
One of the things we’ve learned about the cleverness that resides outside the cranium is that things like the spinal cord are incredibly sophisticated “brains” operating sometimes without much input from upstairs. Through some old experiments that are better not gone into, scientists showed that animals can walk with little brain beyond the parts that regulate circulation and breathing and their spinal cord. This is because the spinal cord can do most of what we need for basic locomotion without any input. The point is that control of the body is distributed—it doesn’t just live in the brain. The lesson hasn’t been lost on robotics folks; for example, Rodney Brooks popularized an approach called “subsumption architecture” based on this idea. So – back to Caprica: For episode 2, “Rebirth,” the show needed some explanation for why the metacognitive processor was only working in one robot. The real reason, as we know, is that only one had Zoe in it; but the roboticists were being pressed by Daniel Graystone as to why it wasn’t working in others. The idea that I gave them, which they used, was that it was because this particular metacognitive processor had distributed its control to peripheral subunits. Because of this, it had become tied to one particular robot. It’s an idea straight out of contemporary neuroscience and efforts to emulate this in robotics.

SPhD: To me, one of the most fascinating directions of the show is the idea that the first Cylon prototype was born of blood, in this case Zoe Graystone, and because of that, carries sentient emotions and thoughts. What is the fine line between a very smart, capable robot and an actual being?
MM: To vastly oversimplify things, you can imagine a gradation in “being” from a rock to a fully sentient self-aware entity. Some of the differentiators between the rock and you include things like the impact of others on how you think about yourself. For example, categorizing a rock as a particular kind of rock has no effect on the constitution of the rock. This isn’t so for self-aware creatures: once a person is labeled a child abuser, it actually affects the constitution of the person so labeled. People treat child abusers differently from non-child abusers. People who are categorized in this way suddenly see themselves differently; and those who were victims do so as well. The philosopher Ian Hacking, who I studied with during my Masters in Philosophy at the University of Toronto, called this the difference between “Human Kinds” and “Natural Kinds.” Another differentiator is that, for what you refer to as an “actual being,” there is a sense of self-interest in continued survival. Because of this, such a being is susceptible to being harmed, and may also therefore have what an ethicist would call “moral worthiness.” Moral worthiness in turn imposes certain obligations in regard to ethical treatment. For example, returning to the rock, we wouldn’t say we harm a rock when we explode it with dynamite, and we wouldn’t accuse the person who did the blowing up of unethical behavior (certain stripes of environmentalism would differ on this point). Unlike a rock, all animals exhibit an interest in self-preservation.
A very smart and capable robot can be imagined which is not affected by how it is categorized by others, and does not have an interest in self-preservation. So, it would fall short of at least those criteria for full-on “being.” But, there’s a lot more that can be said here, of course.
SPhD: What aspect of the Cylon machine and their story, which is now at the heart of Caprica, do you find the most captivating, either as a viewer or a robotics engineer?
MM: The scenario of our inventions eventually becoming so complex that they begin to have an interest in self preservation, and thus can be harmed (and so may start to be candidates for ethical treatment), is one I’ve thought a lot about in the past. It’s a key theme of the show, too. That’s one aspect that fascinates me about the show. The other is the play between the different kinds of being that Zoe has—from avatar-in-a-robot, to avatar-in-virtual reality, to “really real.” It’s a fun fugue on the varieties of being that raises good questions about the nature of existence and mortality, among others.
SPhD: In your latest post for the Science in Society blog, you explore the theoretical question of whether the United States (or any country, for that matter) could develop a Cylon type of war machine. Do you feel there is a distinct possibility the military might ever pursue this option and how might it impact warfare strategy?
MM: I’m going to explore that in my next few posts—and I’m still formulating my thoughts. In their initial development, a more realistic metaphor for how such robot warriors will work with us is something like a dumbed-down well-trained animal, willing to follow commands but without much of a sense of what to do if something gets in the way, and little recourse to things like flexibly generating new behaviors like a real animal does. But I can’t foresee any significant barriers to the development of autonomous robots with more of the attributes of a fuller kind of being I mentioned to above. I feel the more relevant question is whether this is on the order of 10 years away, or a hundred. Once it happens, the question will then be whether the global community recognizes military applications of this technology as a potential threat in need of careful control, like nuclear arms, or not. That’s all for now – for more you’ll have to visit my blog!

SPhD: During last year’s World Science Festival, we covered a really interesting panel called Battlestar Galactica: Cyborgs on the Horizon, which included a cross-section of engineers, ethicists and Battlestar actors discussing artificial intelligence, robotics, and the capabilities of modern engineering, which in some cases are very impressive indeed. In your opinion, what is one of the most significant or promising advance in robotics of the last few years?
MM: The maturing of what is sometimes called “probabilistic robotics.” This approach is what allowed the autonomous car Stanley to win the DARPA Grand Challenge, the challenge to have a vehicle drive itself with no human involvement over a challenging course in the desert. The basic idea is that while traditional robotics was concerned with making precise motions based on very well characterized inputs, what we need for robots to work in the real world is ways to handle the massive array of noisy and uncertain signals that are typically available to guide behavior. There are approaches from probability and statistics for doing this well. These approaches are integral to making robots have greater sensory intelligence. My own laboratory has developed a new kind of sensory robot using this approach, and it works very well.
SPhD: I have previously argued that television and film do more for promoting science by incorporating small, accurate pieces into an overarching story rather than basing an entire story on an unsustainable or far-reaching scientific concept. Thoughts?
MM: TV and film, when it is successful, is about telling a captivating story. The elements of good story telling (emotional connection to the characters, humor, insight into what it is to be human) have little in common with the elements of a good scientific concept (testability, explanatory power, coherence with the rest of what we know). So, yes I’d agree. Trying to do more than incorporate small bits is going to lead to your audience feeling like they are getting a lecture rather having a story shared with them, and no story teller should do that. Documentaries are an interesting hybrid, though—you need a good story, and if it’s about science, a good bit will be on the concepts. How to make that exciting and not spin out into yawn-provoking pedantry is quite the trick.
Malcolm MacIver, PhD, is a professor at Norwestern University with joint appointments in the Biomedical and Mechanical Engineering departments, and an adjunct appointment in the Department of Neurobiology and Physiology. He received a B.Sc. and MA at the University of Toronto, and a PhD in Neuroscience at the University of Illinois in Urbana-Champaign in 2001.
~*ScriptPhD*~
*****************
ScriptPhD.com covers science and technology in entertainment, media and advertising. Hire our consulting company for creative content development.
Follow us on Twitter and our Facebook fan page. Subscribe to free email notifications of new posts on our home page.
]]>
